Decoding Iloperidone: A Comprehensive Study of its R&D Trends
Iloperidone's R&D Progress
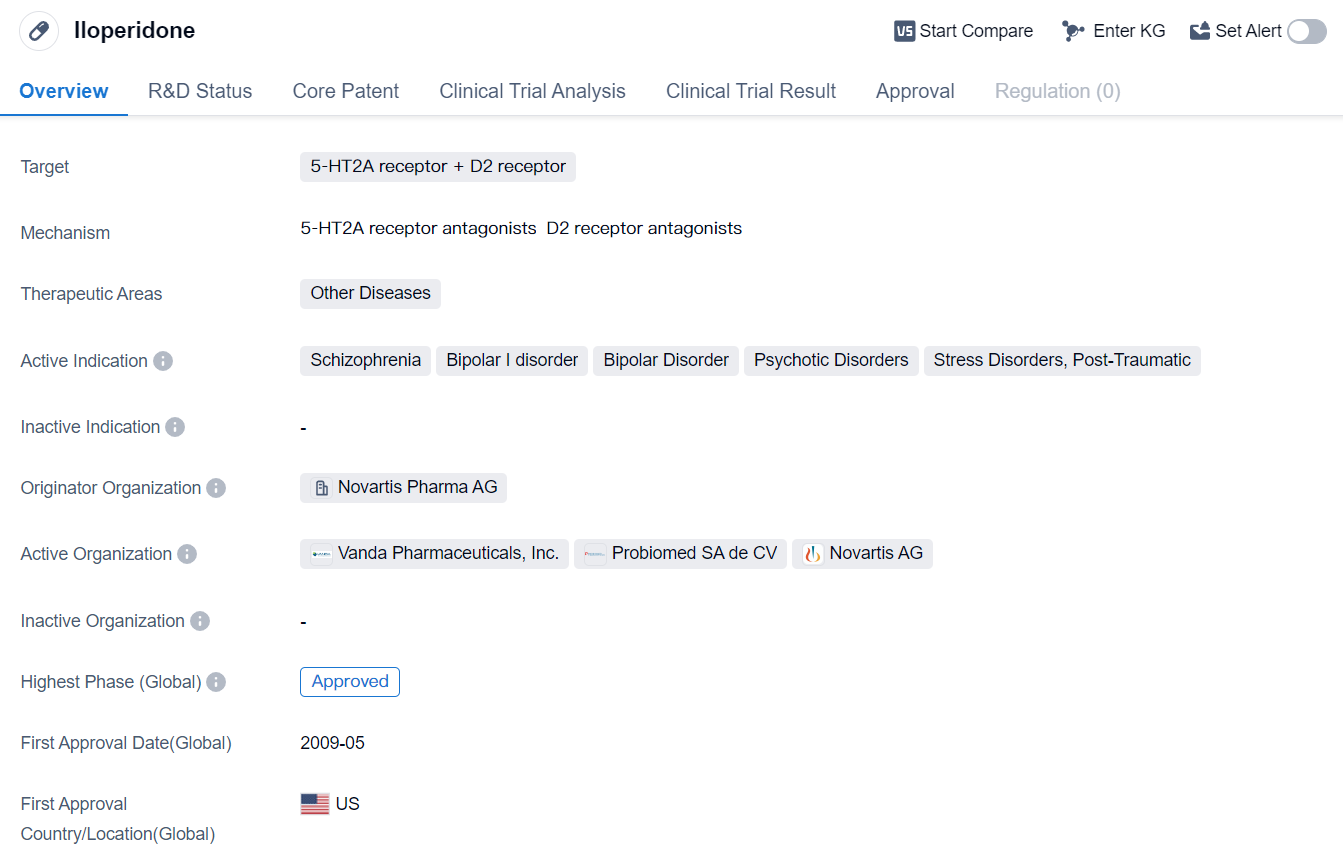
Iloperidone is a small molecule drug that targets both the 5-HT2A receptor and the D2 receptor. It falls under the therapeutic area of Other Diseases and is primarily indicated for the treatment of various mental health disorders such as schizophrenia, bipolar I disorder, bipolar disorder, psychotic disorders, and stress disorders, including post-traumatic stress disorder (PTSD).
The drug was developed by Novartis Pharma AG, a renowned pharmaceutical company. It has successfully completed all phases of clinical trials and has received approval for use in multiple countries. The highest R&D phase of this drug is approved.
The first approval of Iloperidone took place in the United States in May 2009. This signifies that the drug was first authorized for commercial distribution and use in the US market. It is important to note that the information provided is based on factual data and does not include any fictional details or subjective interpretations.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Iloperidone: 5-HT2A receptor antagonist and D2 receptor antagonist
The text refers to two types of receptor antagonists: 5-HT2A receptor antagonists and D2 receptor antagonists.
From a biomedical perspective, receptor antagonists are drugs that bind to specific receptors in the body and prevent the receptor from being activated by its natural ligand. In this case, 5-HT2A receptor antagonists block the activity of the 5-HT2A receptor, which is a subtype of serotonin receptor involved in various physiological processes in the central nervous system. These antagonists can be used in the treatment of psychiatric disorders, such as schizophrenia and depression.
On the other hand, D2 receptor antagonists block the activity of the D2 receptor, which is a subtype of dopamine receptor. Dopamine is a neurotransmitter involved in regulating movement, emotion, and cognition. D2 receptor antagonists are commonly used as antipsychotic medications to treat conditions like schizophrenia and bipolar disorder.
Both 5-HT2A receptor antagonists and D2 receptor antagonists play important roles in modulating neurotransmitter signaling pathways in the brain. By blocking these receptors, these drugs can have therapeutic effects in the treatment of various psychiatric disorders.
Drug Target R&D Trends for Iloperidone
The analysis of the target 5-HT2A receptor + D2 receptor reveals a competitive landscape with several companies showing significant growth and R&D progress. Sumitomo Chemical Co., Ltd., Otsuka Holdings Co., Ltd., AbbVie, Inc., Novartis AG, and Johnson & Johnson are among the companies with the highest number of drugs in various stages of development.
The most common indications for approved drugs targeting this target are schizophrenia, bipolar disorder, depressive disorder, major depressive disorder, and agitation. Small molecule drugs dominate the drug types, indicating intense competition and potential for biosimilars.
The United States, Japan, and the European Union are leading in drug development, with China also making progress. This indicates a global effort in advancing the therapeutic options for the 5-HT2A receptor + D2 receptor target.
Overall, the target 5-HT2A receptor + D2 receptor presents a promising area for drug development, with ongoing research and development efforts to explore its potential for various indications. The competitive landscape is dynamic, and future developments in this field are expected to contribute to improved treatment options for patients.
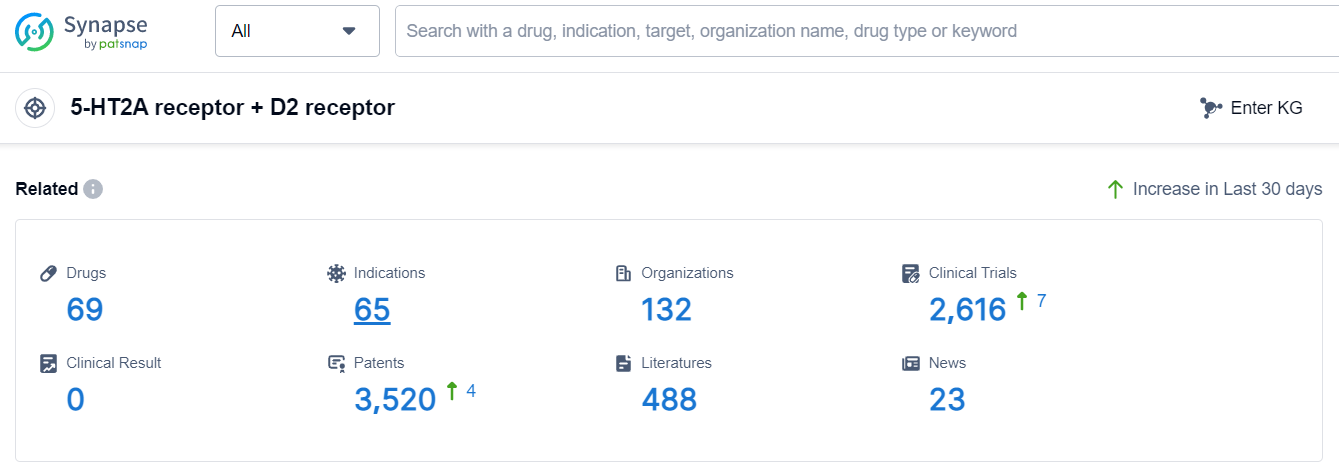
According to Patsnap Synapse, as of 10 Sep 2023, there are a total of 69 5-HT2A receptor + D2 receptor drugs worldwide, from 132 organizations, covering 65 indications, and conducting 2616 clinical trials.
Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Iloperidone is a small molecule drug developed by Novartis Pharma AG. It targets the 5-HT2A receptor and the D2 receptor and is primarily used for the treatment of mental health disorders such as schizophrenia, bipolar I disorder, bipolar disorder, psychotic disorders, and stress disorders, including PTSD. The drug has successfully completed all clinical trial phases and has received approval for use in multiple countries. Its first approval occurred in the United States in May 2009.