Deep Scientific Insights on Tucatinib's R&D Progress
Tucatinib's R&D Progress
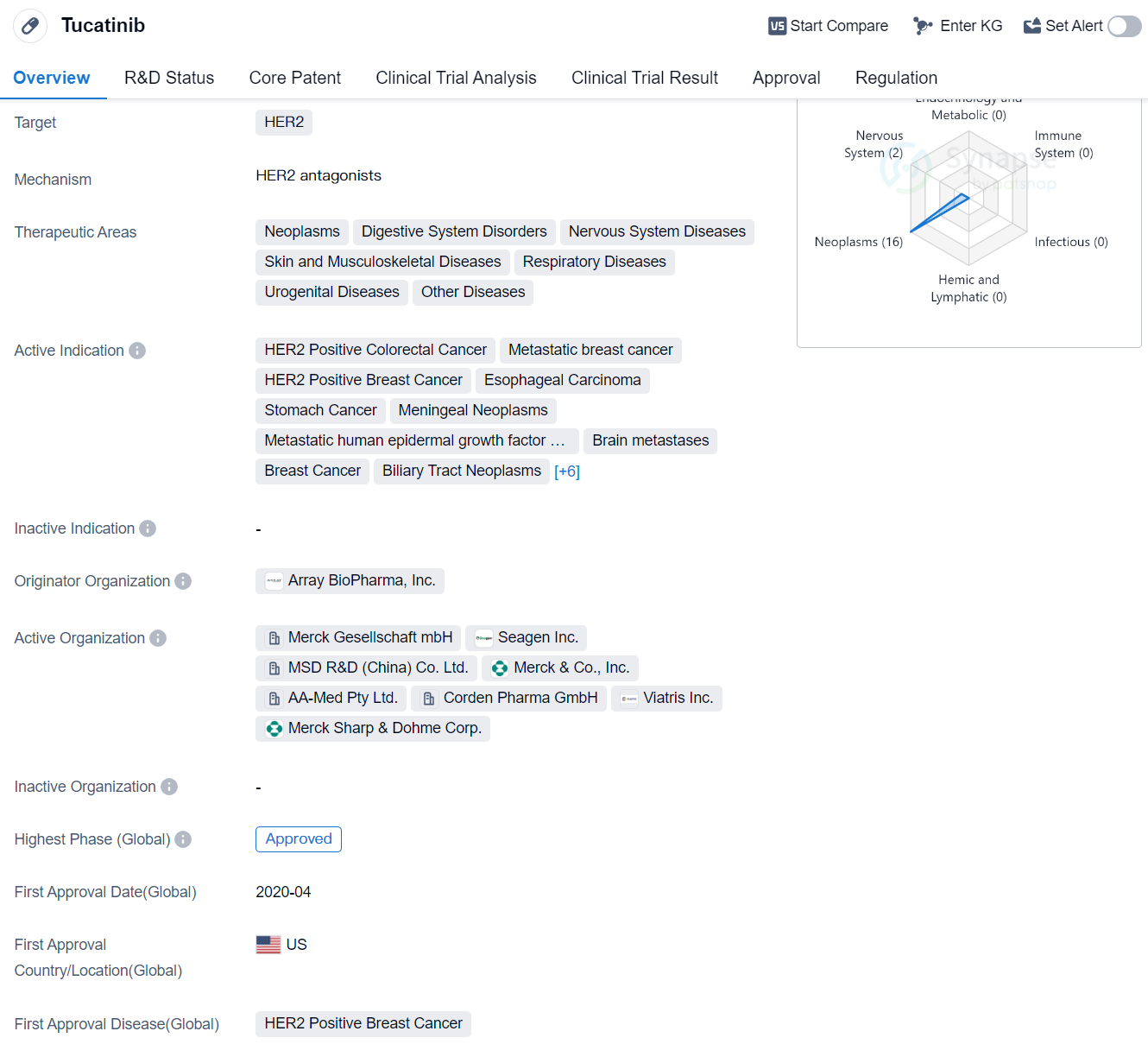
Tucatinib is a small molecule drug that falls under the category of biomedicine. It primarily targets HER2, a protein that is overexpressed in certain types of cancers. The drug has shown potential therapeutic benefits in various therapeutic areas, including neoplasms, digestive system disorders, nervous system diseases, skin and musculoskeletal diseases, respiratory diseases, urogenital diseases, and other diseases.
Tucatinib has been indicated for the treatment of several types of cancers, including HER2 positive colorectal cancer, metastatic breast cancer, HER2 positive breast cancer, esophageal carcinoma, stomach cancer, meningeal neoplasms, metastatic human epidermal growth factor 2 positive carcinoma of breast, brain metastases, breast cancer, biliary tract neoplasms, metastatic non-small cell lung cancer, uterine cervical cancer, cholangiocarcinoma, gallbladder neoplasms, gastroesophageal junction adenocarcinoma, and stomach adenocarcinoma.
The drug was developed by Array BioPharma, Inc., an originator organization in the pharmaceutical industry. Tucatinib has reached the highest phase of development, which is approved globally. In China, it is currently in phase 3 of development.
Tucatinib received its first approval in the United States in April 2020. The drug has undergone regulatory processes such as priority review, accelerated approval, fast track designation, orphan drug designation, and breakthrough therapy designation. These regulatory designations highlight the potential significance and urgency of Tucatinib in addressing unmet medical needs.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Tucatinib: HER2 antagonist
From a biomedical perspective, HER2 antagonists are a type of medication that specifically target and inhibit the activity of the HER2 protein. HER2 (human epidermal growth factor receptor 2) is a receptor protein that is overexpressed in certain types of cancer, such as breast cancer. Overexpression of HER2 can lead to uncontrolled cell growth and division, contributing to the progression of cancer.
HER2 antagonists work by binding to the HER2 protein and blocking its signaling pathway, thereby preventing the growth and spread of cancer cells. These antagonists can be in the form of monoclonal antibodies, small molecules, or bispecific antibodies. Monoclonal antibodies, such as trastuzumab and pertuzumab, are commonly used HER2 antagonists in the treatment of HER2-positive breast cancer.
By inhibiting the HER2 protein, HER2 antagonists help to disrupt the growth signals that promote cancer cell proliferation. They can be used as targeted therapies either alone or in combination with other cancer treatments, such as chemotherapy or hormone therapy. The use of HER2 antagonists has significantly improved the outcomes for patients with HER2-positive breast cancer, leading to increased survival rates and better disease control.
Drug Target R&D Trends for Tucatinib
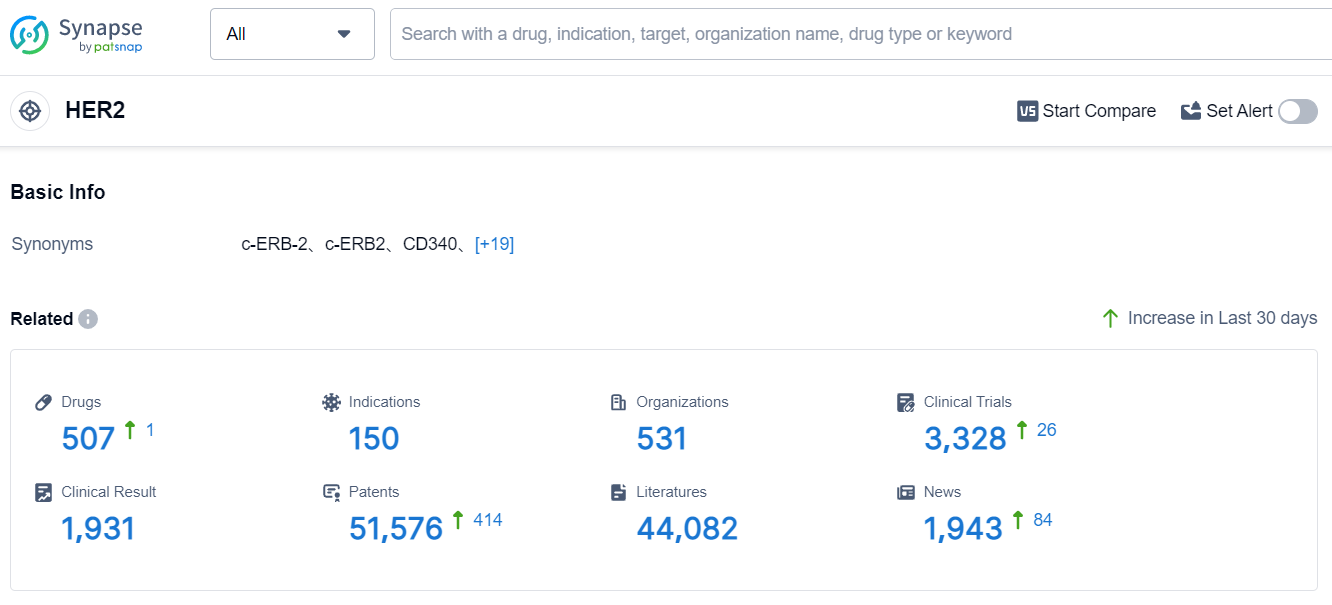
According to Patsnap Synapse, as of 4 Sep 2023, there are a total of 507 HER2 drugs worldwide, from 531 organizations, covering 150 indications, and conducting 3328 clinical trials.
The analysis of the current competitive landscape of target HER2 reveals that Roche Holding AG is the leading company with the highest stage of development. Several companies, including Pfizer Inc., Daiichi Sankyo Co., Ltd., and Shanghai Fosun High Technology (Group) Co., Ltd., are also actively involved in the research and development of drugs targeting HER2. The approved indications for drugs under this target include various types of cancer, such as breast cancer, gastric cancer, lung cancer, and colorectal cancer. Monoclonal antibody and biosimilar are the most rapidly progressing drug types, indicating intense competition. The United States and China are the leading countries in terms of drug development, with significant progress in both regions. The progress in China suggests a promising future for the pharmaceutical industry in the country. Overall, the target HER2 presents a competitive landscape with multiple companies and drug types, indicating a focus on developing innovative therapies for various indications.
Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Tucatinib is a small molecule drug developed by Array BioPharma, Inc. It targets HER2 and has shown promise in treating various types of cancers. The drug has received approval in the United States and is currently in phase 3 development in China. Its regulatory designations emphasize its potential as a breakthrough therapy for patients with limited treatment options.