Important Target in Synthetic Lethality: ATR Inhibitors
The Ataxia telangiectasia and Rad-3 related protein kinase (ATR) is a member of the PIKK protein kinase family, and it is involved in numerous complex DNA damage repair mechanisms. Consequently, these mechanisms help to maintain genome integrity when the body deals with various genomic insults.
Once DNA is damaged, a post-translational modification network can be activated to initiate damage checkpoints, subsequently inducing DNA repair and cellular apoptosis, a process known as the DNA damage response (DDR). ATR has the ability to activate cellular responses following DNA damage and can halt cell cycle progression, stabilize replication forks, and repair DNA. This process not only circumvents cell apoptosis but also plays an essential role in life activities. As a type of DNA damage response mechanism, the ATR pathway plays a crucial inhibitory role in tumor survival. Inducing ATR pathway-dependent malignant tumor cell death serves as an ideal target for developing low-toxicity, high-efficacy targeted anti-tumor drugs.
Synthetic lethality refers to a scenario where a mutation in either of two non-lethal genes does not affect cell survival, but simultaneous mutations in both genes can specifically trigger cell death. Research has found that ATM and ATR kinases are activated or upregulated in cancer cells, and ATR serves as a synthetic lethal target for ATM mutations. Tumor cells deficient in ATM are more susceptible to ATR inhibitors. The selective inhibition between the two offers a new approach to tumor treatment and provides a new tool for tumor research.
ATR Competitive Landscape
According to the data provided by Patsnap Synapse-Global Drug Intelligence Database: the following figure shows that as of 8 Sep 2023, there are a total of 24 ATR drugs worldwide, from 36 organizations, covering 45 indications, and conducting 127 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.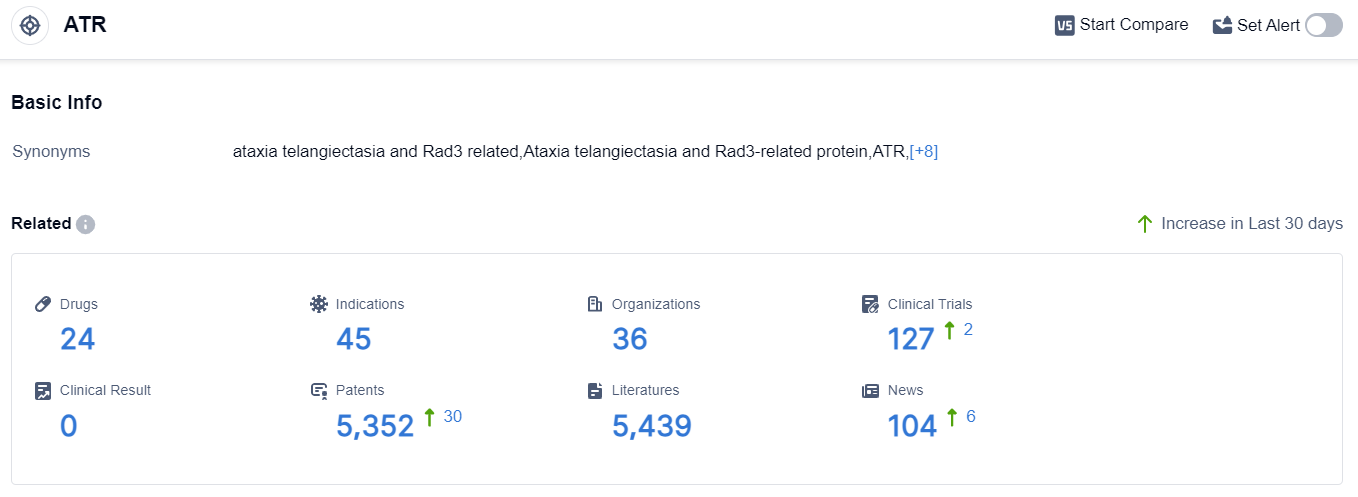
The current competitive landscape of target ATR in the pharmaceutical industry is dominated by companies like AstraZeneca PLC, Merck KGaA, and National Cancer Institute. These companies have a significant number of drugs in various development phases, indicating their commitment to research and development in this area.
The approved indications for drugs under the target ATR cover a wide range of cancers, highlighting the potential impact of these drugs in treating different types of cancer. The rapid progress of small molecule drugs suggests intense competition in the development of innovative drugs. However, the presence of biosimilars is not mentioned in the provided data.
The United States, China, and the European Union are the leading countries/locations in terms of development under the target ATR, with China showing notable progress. Overall, the target ATR presents a promising opportunity for the pharmaceutical industry, with a focus on cancer treatment and the development of small molecule drugs.
Phase III Clinical Trial of ATR Inhibitor: Ceralasertib
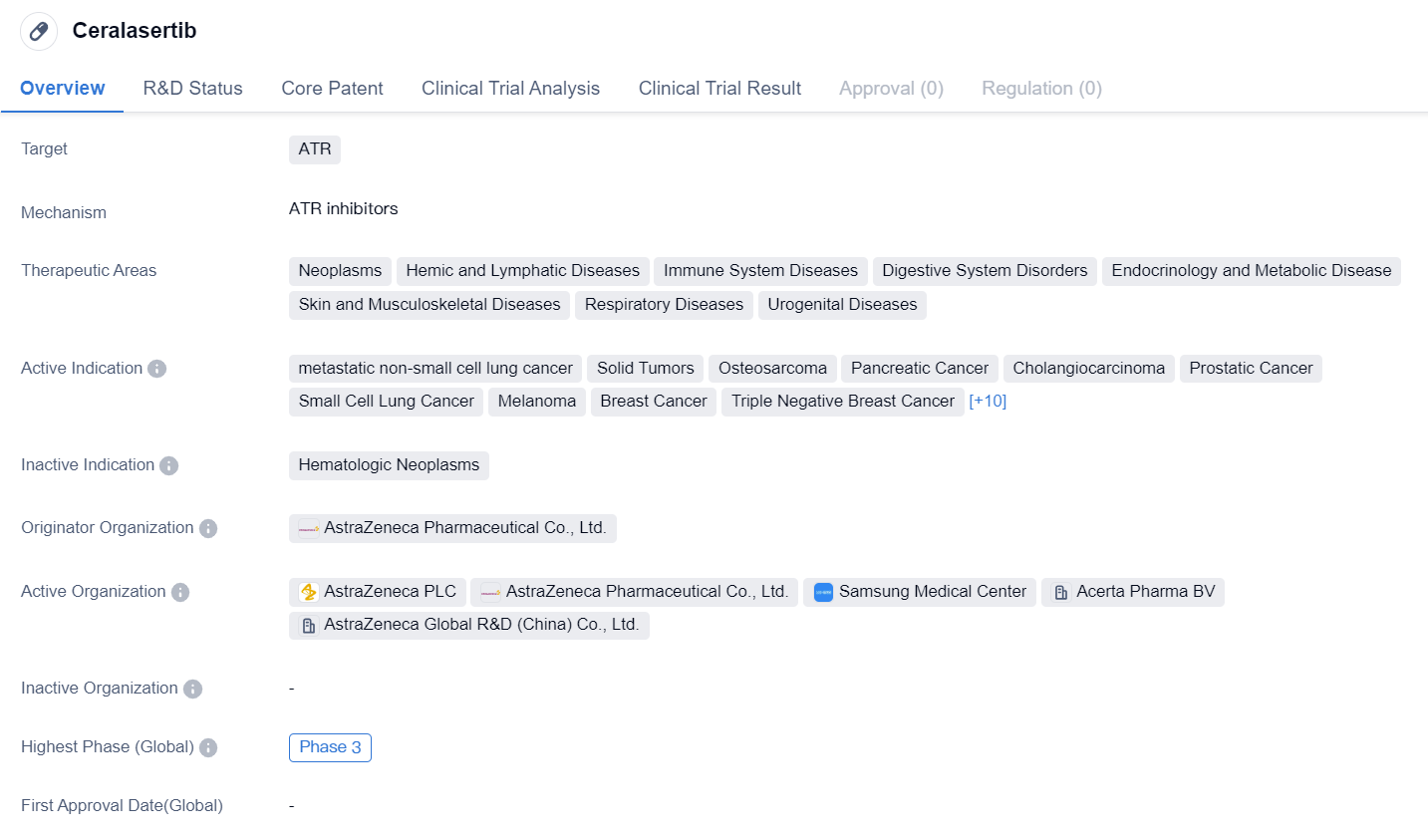
Ceralasertib is a small molecule drug that targets the protein ATR. It is being developed by AstraZeneca Pharmaceutical Co., Ltd. and is currently in Phase 3 clinical trials, both globally and in China.
The drug has shown potential in treating a wide range of therapeutic areas, including neoplasms, hemic and lymphatic diseases, immune system diseases, digestive system disorders, endocrinology and metabolic diseases, skin and musculoskeletal diseases, respiratory diseases, and urogenital diseases.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
In terms of active indications, Ceralasertib has demonstrated efficacy in metastatic non-small cell lung cancer, solid tumors, osteosarcoma, pancreatic cancer, cholangiocarcinoma, prostatic cancer, small cell lung cancer, melanoma, breast cancer, triple negative breast cancer, B-cell chronic lymphocytic leukemia, non-small cell lung cancer, ovarian cancer, squamous cell carcinoma, stomach cancer, chronic myelogenous leukemia, diffuse large B-cell lymphoma, myelodysplastic syndromes, chronic myelomonocytic leukemia, and squamous cell carcinoma of the head and neck.
Phase 3 is the highest phase of clinical development, indicating that Ceralasertib has progressed through earlier stages of testing and has shown promising results in terms of safety and efficacy. This phase involves large-scale clinical trials to further evaluate the drug's effectiveness and monitor any potential side effects.
Phase II Clinical Trial of ATR Inhibitor: Berzosertib
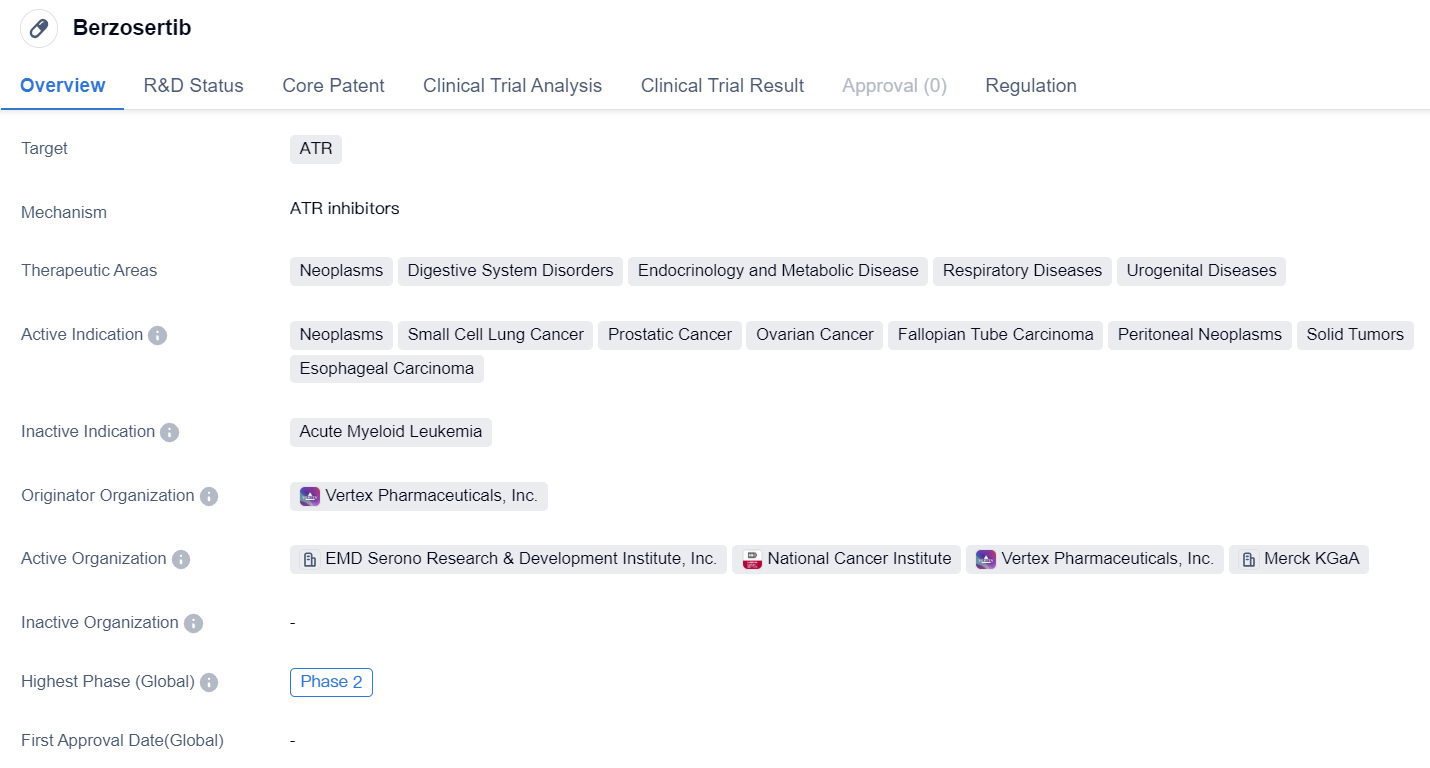
Berzosertib is a small molecule drug that targets the protein ATR. It is being developed for the treatment of various therapeutic areas including neoplasms, digestive system disorders, endocrinology and metabolic disease, respiratory diseases, and urogenital diseases. The drug is specifically indicated for neoplasms, small cell lung cancer, prostatic cancer, ovarian cancer, fallopian tube carcinoma, peritoneal neoplasms, solid tumors, and esophageal carcinoma.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Berzosertib is being developed by Vertex Pharmaceuticals, Inc., a renowned originator organization in the pharmaceutical industry. Currently, the drug has reached Phase 2 of clinical development. This indicates that it has already undergone initial testing for safety and efficacy in a limited number of patients.
One notable aspect of Berzosertib is its regulatory status as an orphan drug. Orphan drugs are medications developed to treat rare diseases or conditions that affect a small number of patients. This designation provides certain incentives to the pharmaceutical company, such as extended market exclusivity and financial support for research and development.
Based on the available information, Berzosertib shows promise in the field of biomedicine. Its target, ATR, is involved in DNA damage response and repair, making it a potential candidate for the treatment of various types of cancer. The drug's active indications, including small cell lung cancer, prostatic cancer, and ovarian cancer, highlight its potential in addressing significant unmet medical needs in these areas.
However, it is important to note that the information provided is limited, and further research and clinical trials are necessary to fully evaluate the safety and efficacy of Berzosertib. The drug's current phase of development suggests that it is still in the early stages of testing, and its ultimate success will depend on the results of future studies.
In conclusion, Berzosertib is a small molecule drug developed by Vertex Pharmaceuticals, Inc. that targets ATR. It is being investigated for its potential in treating various neoplasms and has reached Phase 2 of clinical development. As an orphan drug, it holds promise for addressing unmet medical needs in rare diseases. However, additional research is needed to determine its safety and efficacy.