Decoding Rocuronium Bromide: A Comprehensive Study of its R&D Trends
Rocuronium Bromide's R&D Progress
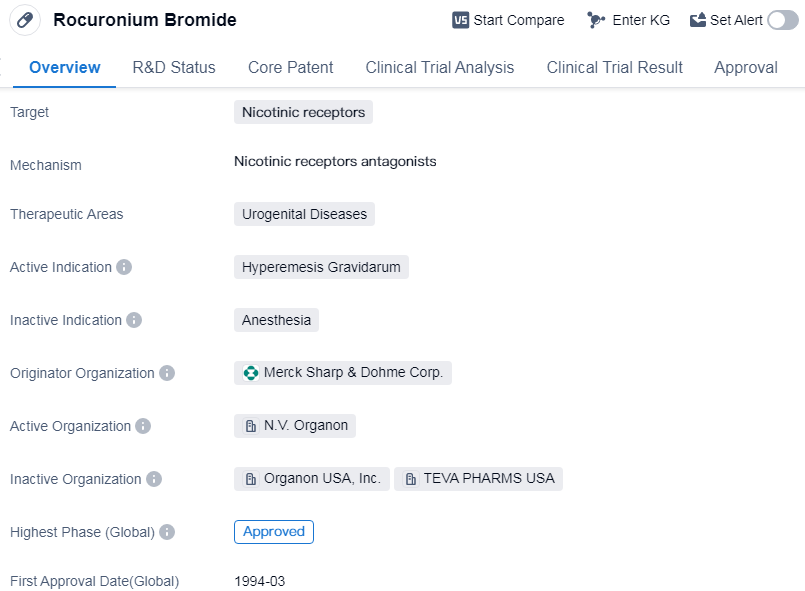
Rocuronium Bromide is a small molecule drug that targets nicotinic receptors. It is primarily used in the treatment of urogenital diseases, specifically hyperemesis gravidarum. The drug was developed by Merck Sharp & Dohme Corp., a renowned pharmaceutical organization.
Rocuronium Bromide has successfully completed the approval process and has been granted global approval. The highest phase achieved by this drug is approved, indicating that it has undergone rigorous testing and evaluation to ensure its safety and efficacy.
The first approval of Rocuronium Bromide took place in March 1994 in the United States. The approval in the United States suggests that it has met the necessary regulatory requirements and demonstrated its effectiveness in treating hyperemesis gravidarum.
Hyperemesis gravidarum is a condition characterized by severe nausea and vomiting during pregnancy. It is considered a urogenital disease as it affects the urinary and reproductive systems. Rocuronium Bromide with its specific targeting of nicotinic receptors, aims to alleviate the symptoms associated with hyperemesis gravidarum and improve the quality of life for affected individuals.
As a small molecule drug, Rocuronium Bromide is likely to have a well-defined chemical structure, allowing for precise understanding of its mechanism of action. This can aid in the development of potential derivatives or modifications to enhance its therapeutic effects or reduce any potential side effects.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Rocuronium Bromide: Nicotinic receptors antagonists
Nicotinic receptors antagonists are a class of drugs that block the action of nicotinic acetylcholine receptors in the body. These receptors are located in the central and peripheral nervous systems and are involved in various physiological processes, including neurotransmission.
From a biomedical perspective, nicotinic receptors antagonists are commonly used in the treatment of conditions related to the overactivation of nicotinic receptors, such as nicotine addiction and certain neurological disorders. By blocking the receptors, these drugs can help reduce the effects of nicotine or regulate abnormal neurotransmission, leading to therapeutic benefits.
Nicotinic receptors antagonists can have different mechanisms of action and can be classified into various subtypes based on their selectivity and binding properties. Some examples of nicotinic receptors antagonists include mecamylamine, varenicline, and bupropion. These drugs may be administered orally or through other routes, depending on the specific indication and formulation.
It is important to note that the use of nicotinic receptors antagonists should be done under medical supervision, as these drugs can have side effects and interactions with other medications. The dosage and duration of treatment may vary depending on the individual and the condition being treated.
In summary, nicotinic receptors antagonists are drugs that block the action of nicotinic acetylcholine receptors in the body, and they are used in the treatment of conditions related to nicotinic receptor overactivation.
Drug Target R&D Trends for Rocuronium Bromide
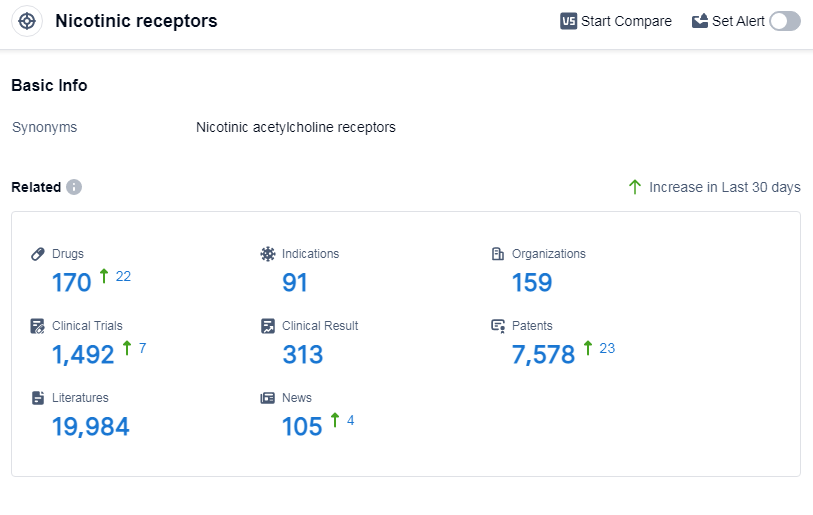
According to Patsnap Synapse, as of 8 Sep 2023, there are a total of 170 nicotinic receptors drugs worldwide, from 159 organizations, covering 91 indications, and conducting 1492 clinical trials.
The analysis of target nicotinic receptors reveals a competitive landscape with multiple companies involved in the development of drugs. Pfizer Inc. stands out with the highest number of drugs and significant R&D progress. The approved drugs target various indications such as hyperemesis gravidarum, anesthesia, pain, smoking cessation, and etc. Small molecule drugs dominate the development pipeline, but there is also representation from biological products, diagnostic radiopharmaceuticals, and other drug types. The United States, China, and the European Union are the leading countries/locations in terms of drug development. China has shown progress with 7 approved drugs. Overall, the target nicotinic receptors present opportunities for therapeutic advancements and competition in the pharmaceutical industry.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
Overall, Rocuronium Bromide is an approved drug developed by Merck Sharp & Dohme Corp. It targets nicotinic receptors and is primarily used in the treatment of hyperemesis gravidarum, which is a urogenital disease. With its first approval in the United States in 1994, Rocuronium Bromide has established its efficacy and safety profile, making it a valuable option for healthcare professionals in managing this condition.