Pholcodine: Detailed Review of its Transformative R&D Success, Mechanism of Action, and Drug Target
Pholcodine's R&D Progress
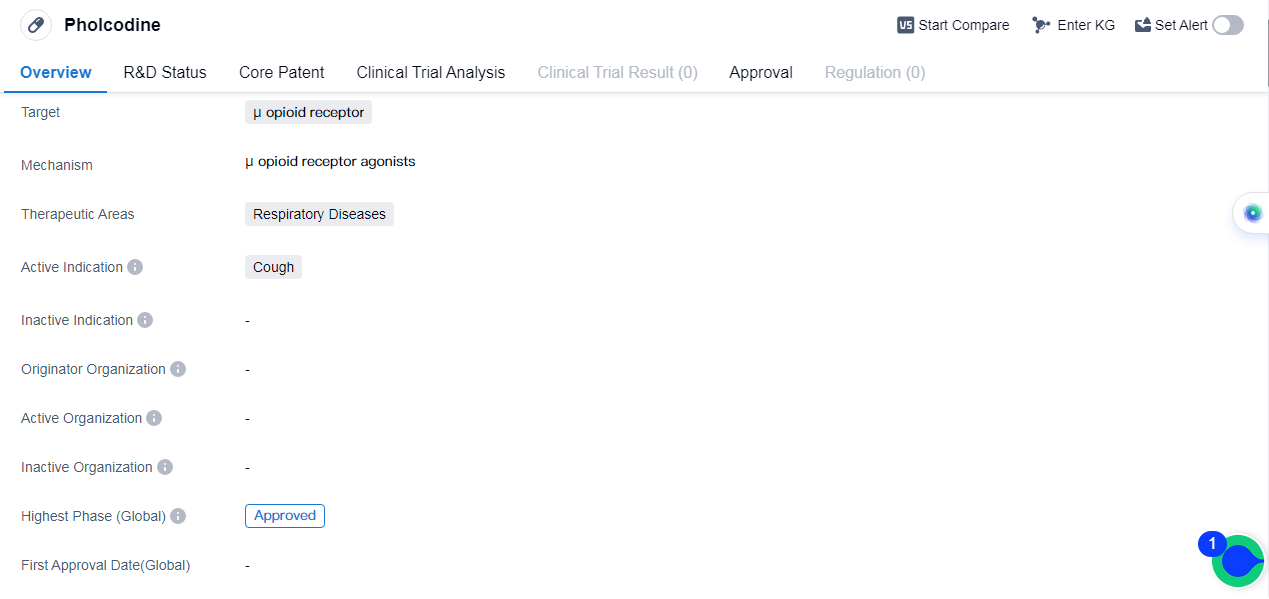
Pholcodineis a small molecule drug that targets the μ opioid receptor. It is primarily used in the treatment of respiratory diseases, specifically for alleviating cough symptoms. The drug has reached the highest phase of approval globally.
As a small molecule drug, Pholcodine is composed of low molecular weight compounds that can easily penetrate cell membranes. This characteristic allows for efficient drug delivery and interaction with the μ opioid receptor, which is the target of Pholcodine. By binding to this receptor, the drug modulates the body's response to coughing, reducing its frequency and severity, andproviding relief to patients suffering from respiratory diseases.
Pholcodine's therapeutic areas are focused on respiratory diseases, indicating its potential effectiveness in treating conditions such as bronchitis, asthma, and other respiratory tract infections.
The fact that Pholcodine has reached the highest phase of approval globally indicates that it has undergone rigorous testing and evaluation to ensure its safety and efficacy. This approval status signifies that the drug has met the necessary regulatory requirements and has demonstrated positive results in clinical trials.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Pholcodine: μOpioid Receptor Agonists
From a biomedical perspective, μ opioid receptor agonists are a type of drugs that activate the μ opioid receptors in the body. These receptors are primarily found in the central nervous system and are involved in modulating pain perception, as well as other physiological processes. When μ opioid receptors are activated by agonists, such as certain opioids, they produce analgesic effects by reducing the transmission of pain signals in the brain and spinal cord. This can lead to pain relief and a sense of euphoria. However, it is important to note that μ opioid receptor agonists also carry the risk of dependence, tolerance, and potential for misuse, making them subject to strict regulation and controlled use.
Drug Target R&D Trends for Pholcodine
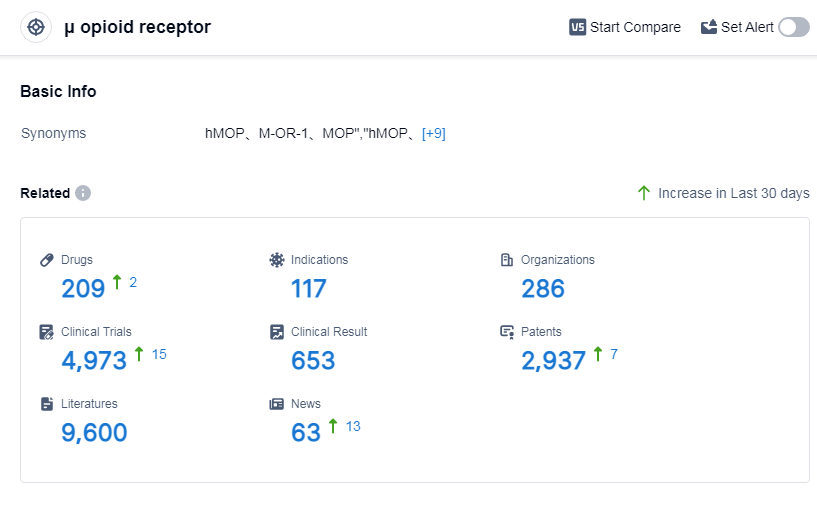
According to Patsnap Synapse, as of 6 Sep 2023, there are a total of 209 μ opioid receptor drugs worldwide, covering 117 indications, and conducting 4973 clinical trials, from 286 organizations.
The analysis of the target μ opioid receptor in the pharmaceutical industry reveals a competitive landscape with several companies making progress in the development of drugs. Johnson & Johnson, Collegium Pharmaceutical, Inc., and Wuhan Dangdai Technology Industry Group Co. Ltd. are among the companies growing the fastest. The highest stage of development is the approved phase, with a focus on pain, such as acute pain and cancer pain. Small molecule drugs are progressing most rapidly, indicating intense competition in this area. China has shown significant progress in the development of drugs targeting the μ opioid receptor. Overall, the future development of this target holds promise for the pharmaceutical industry.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Pholcodine is a small molecule drug that targets the μ opioid receptor and is primarily used in the treatment of respiratory diseases, specifically for alleviating cough symptoms. It has reached the highest phase of approval globally, indicating its potential as an effective treatment option for patients suffering from respiratory conditions.