Deep Scientific Insights on Ripretinib's R&D Progress, Mechanism of Action, and Drug Target
Ripretinib's R&D Progress
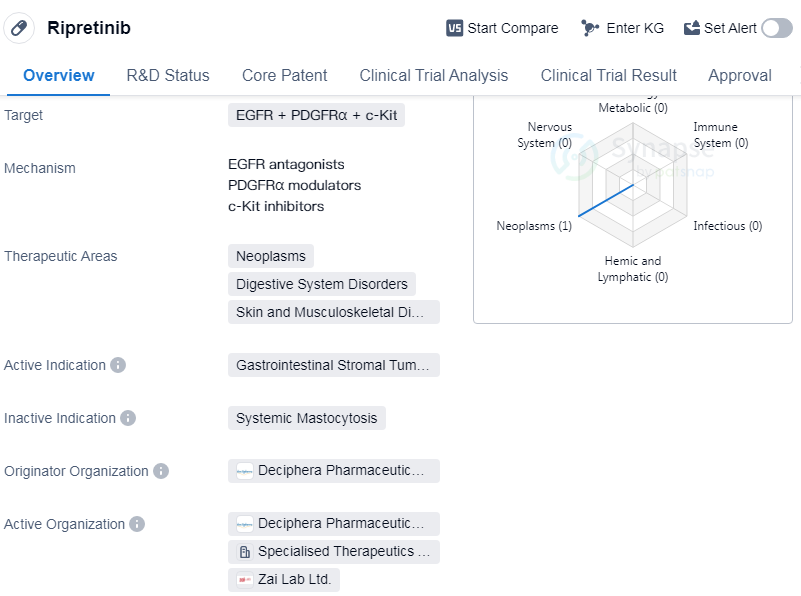
Ripretinib is a small molecule drug that targets epidermal growth factor receptor (EGFR), platelet-derived growth factor receptor alpha (PDGFRα), and c-Kit. It is primarily used in the treatment of gastrointestinal stromal tumors (GISTs). The drug is developed by Deciphera Pharmaceuticals, Inc., a pharmaceutical organization specializing in biomedicine.
Ripretinib has received approval for use in the United States and China, indicating its efficacy and safety in treating GISTs. The drug's first approval was granted in May 2020 in the United States. It is important to note that Ripretinib has undergone various regulatory processes, including priority review, fast-track designation, breakthrough therapy designation, and orphan drug designation.
The therapeutic areas of Ripretinib extend beyond GISTs and include neoplasms (abnormal growth of cells), digestive system disorders, and skin and musculoskeletal diseases.
As a small molecule drug, Ripretinib is designed to interact with specific molecular targets, such as EGFR, PDGFRα, and c-Kit. By targeting these receptors, Ripretinib aims to inhibit their activity and disrupt the signaling pathways involved in tumor growth and progression. This mechanism of action makes Ripretinib a promising option for patients with GISTs, as it directly targets the molecular drivers of the disease.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Ripretinib: EGFR antagonists, PDGFRα modulators and c-Kit inhibitors
EGFR antagonists are a type of medication that specifically target and inhibit the activity of the epidermal growth factor receptor (EGFR). EGFR is a protein found on the surface of cells and plays a crucial role in regulating cell growth and division. By blocking the activation of EGFR, these antagonists help to prevent the excessive growth and proliferation of cells, particularly cancer cells that rely on EGFR signaling for their survival and growth. EGFR antagonists are commonly used in the treatment of various types of cancer, including lung, colorectal, and head and neck cancers.
PDGFRα modulators are medications that modulate or regulate the activity of the platelet-derived growth factor receptor alpha (PDGFRα). PDGFRα is a protein involved in cell signaling and is important for the growth and development of various tissues in the body. By modulating PDGFRα activity, these drugs can have different effects depending on the specific condition being treated. For example, in certain types of cancer, inhibiting PDGFRα may help to suppress tumor growth and metastasis. In other cases, activating PDGFRα may promote tissue repair and regeneration.
c-Kit inhibitors are drugs that specifically target and inhibit the activity of the c-Kit receptor. c-Kit is a type of receptor tyrosine kinase that plays a crucial role in the development and function of certain cells, including stem cells and mast cells. By inhibiting c-Kit, these drugs can interfere with the growth and survival of cells that rely on c-Kit signaling. c-Kit inhibitors are used in the treatment of various conditions, including certain types of cancer (such as gastrointestinal stromal tumors) and certain allergic disorders (such as mastocytosis).
Overall, EGFR antagonists, PDGFRα modulators, and c-Kit inhibitors are all types of medications that target specific receptors involved in cell signaling pathways. By modulating the activity of these receptors, these drugs can have therapeutic effects in various diseases and conditions.
Drug Target R&D Trends for Ripretinib
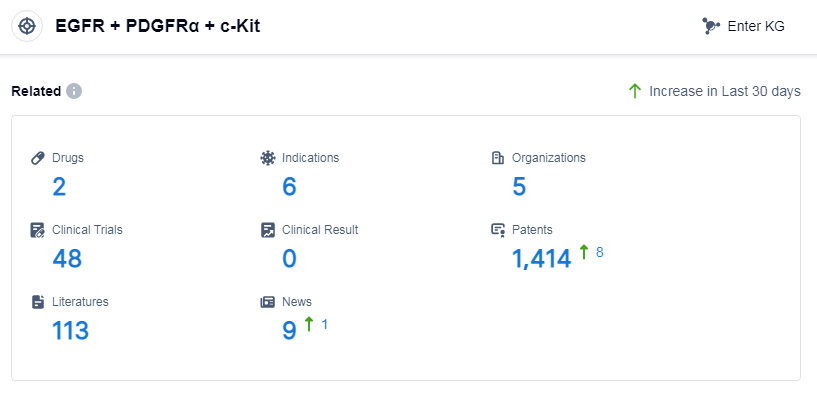
According to Patsnap Synapse, as of 11 Sep 2023, there are a total of 2 EGFR, PDGFRα and c-Kit drugs worldwide, from 5 organizations, covering 6 indications, and conducting 48 clinical trials.
Based on the analysis of the provided data, the target EGFR, PDGFRα and c-Kit shows potential for further development in the pharmaceutical industry. Deciphera Pharmaceuticals, Inc., Specialised Therapeutics Asia Pte Ltd., and Zai Lab Ltd. are the key companies involved in the development of drugs targeting this target. GISTs is the only approved indication, indicating the need for further research on other indications. Small molecule drugs are progressing under this target, with limited competition from biosimilars. Various countries/locations, including China, are actively involved in the development of drugs targeting EGFR, PDGFRα and c-Kit. Overall, the current competitive landscape suggests opportunities for future development and advancements in this target.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
Overall, Ripretinib's approval in both the United States and China, along with its regulatory designations, highlights its potential as a treatment option for GISTs. Its ability to target multiple receptors involved in tumor growth and its potential applications in various therapeutic areas make it a significant development in the field of biomedicine.