Pentosan Polysulfate Sodium Unveiled: A Detailed Overview of its Revolutionary R&D Breakthroughs
Pentosan Polysulfate Sodium's R&D Progress
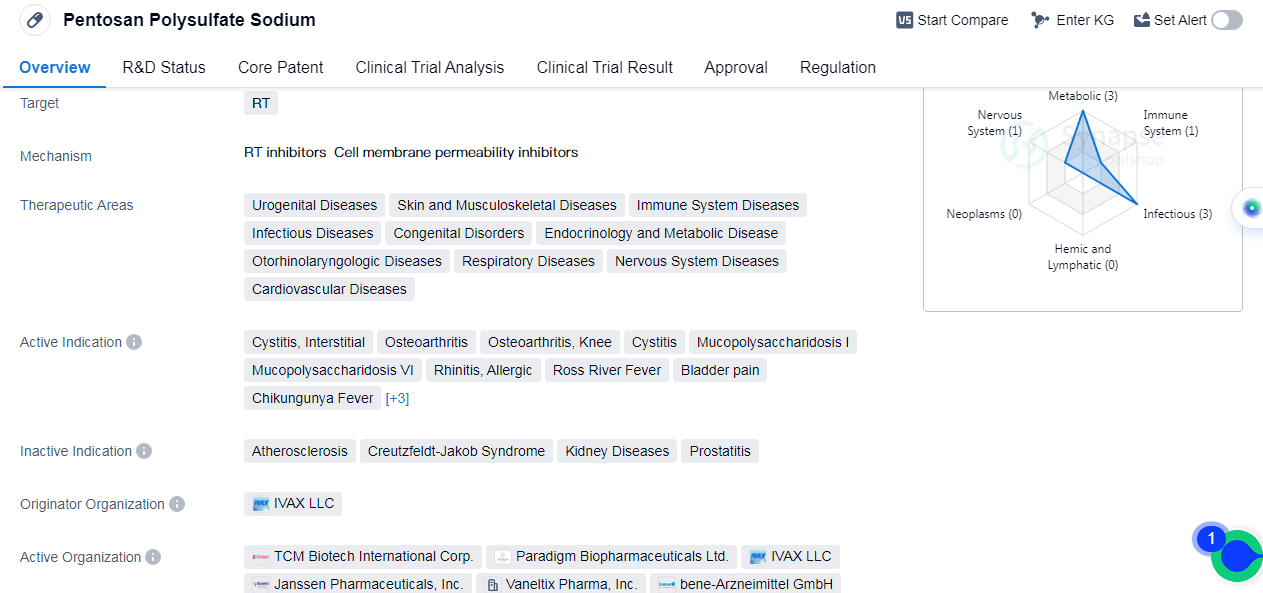
Pentosan Polysulfate Sodium is a small molecule drug that primarily targets the renal tubular (RT) system. It has been approved for use in various therapeutic areas, including urogenital diseases, skin and musculoskeletal diseases, immune system diseases, infectious diseases, congenital disorders, and etc.
The drug is also indicated for the treatment of other conditions such as cystitis, interstitial, osteoarthritis (including knee osteoarthritis), mucopolysaccharidosis I and VI, rhinitis (allergic), Ross River fever,bladder pain, chikungunya fever, chronic heart failure, respiratory distress syndrome (in adults), and mucopolysaccharidoses.
Pentosan Polysulfate Sodium was first approved by the United States in September 1996. The drug's originator organization is IVAX LLC. It has reached the highest phase of development, which is the approved stage.
In terms of regulatory status, Pentosan Polysulfate Sodium has been granted fast-track designation and orphan drug status. Fast-track designation is given to drugs that address unmet medical needs and have the potential to provide significant benefits over existing treatments. Orphan drug status is granted to drugs intended for the treatment of rare diseases.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Pentosan Polysulfate Sodium: RT inhibitors and Cell Membrane Permeability Inhibitors
RT inhibitors and cell membrane permeability inhibitors are two different types of drugs used in biomedicine.
RT inhibitors, also known as reverse transcriptase inhibitors, are a class of antiviral drugs that target the enzyme reverse transcriptase. Reverse transcriptase is an essential enzyme for the replication of retroviruses, such as HIV. By inhibiting this enzyme, RT inhibitors can effectively block the replication of the virus and slow down the progression of the disease. These inhibitors are commonly used in the treatment of HIV/AIDS.
Cell membrane permeability inhibitorsare a type of drug that modulates the permeability of cell membranes. Cell membranes are selectively permeable barriers that regulate the passage of molecules and ions into and out of cells. By targeting specific membrane proteins or channels, cell membrane permeability inhibitors can alter the permeability of the membrane, either by increasing or decreasing it. This modulation of membrane permeability can have various effects, such as altering the transport of nutrients, ions, or drugs across the membrane.
It is important to note that RT inhibitors and cell membrane permeability inhibitors are distinct drug types with different mechanisms of action. While RT inhibitors specifically target viral enzymes, cell membrane permeability inhibitors have a broader scope of action in modulating the permeability of cell membranes.
Drug Target R&D Trends for Pentosan Polysulfate Sodium
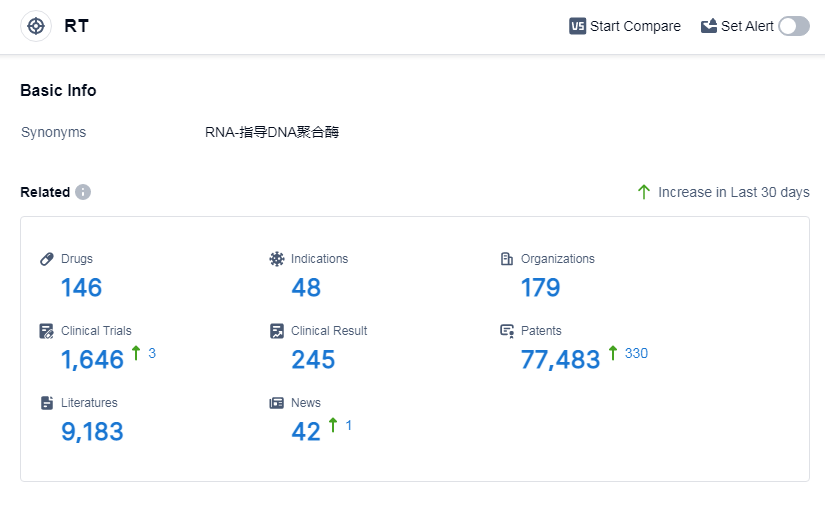
According to Patsnap Synapse, as of 6 Sep 2023, there are a total of 146 RT drugs worldwide, covering 48 indications, and conducting 1646 clinical trials, from 179 organizations.
Based on the analysis of the data provided, the current competitive landscape of target RT is characterized by the presence of several key companies, including Gilead Sciences, Inc., GSK Plc, Johnson & Johnson, and Merck & Co., Inc. These companies have made significant progress in the R&D of drugs targeting RT, with a focus on indications such as HIV Infections, HIV-1 infection, and Hepatitis B. The development of small molecule drugs and synthetic peptides is progressing rapidly, indicating intense competition in the pharmaceutical industry. The countries/locations that are developing fastest under the current targets include the United States, European Union, China, Japan, and Canada, with China showing notable progress. Overall, the future development of target RT is promising, with ongoing research and development efforts aimed at addressing unmet medical needs in various indications.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Pentosan Polysulfate Sodium is a small molecule drug developed by IVAX LLC. It has been approved for use in various therapeutic areas and targets the RT system. The drug has a wide range of indications, including cystitis, osteoarthritis, mucopolysaccharidosis, rhinitis, and respiratory distress syndrome. It received its first approval in the United States in 1996 and has been granted fast-track and orphan drug designations.