Decoding Terbinafine hydrochloride: A Comprehensive Study of its R&D Trends and Mechanism on Drug Target
Terbinafine hydrochloride's R&D Progress
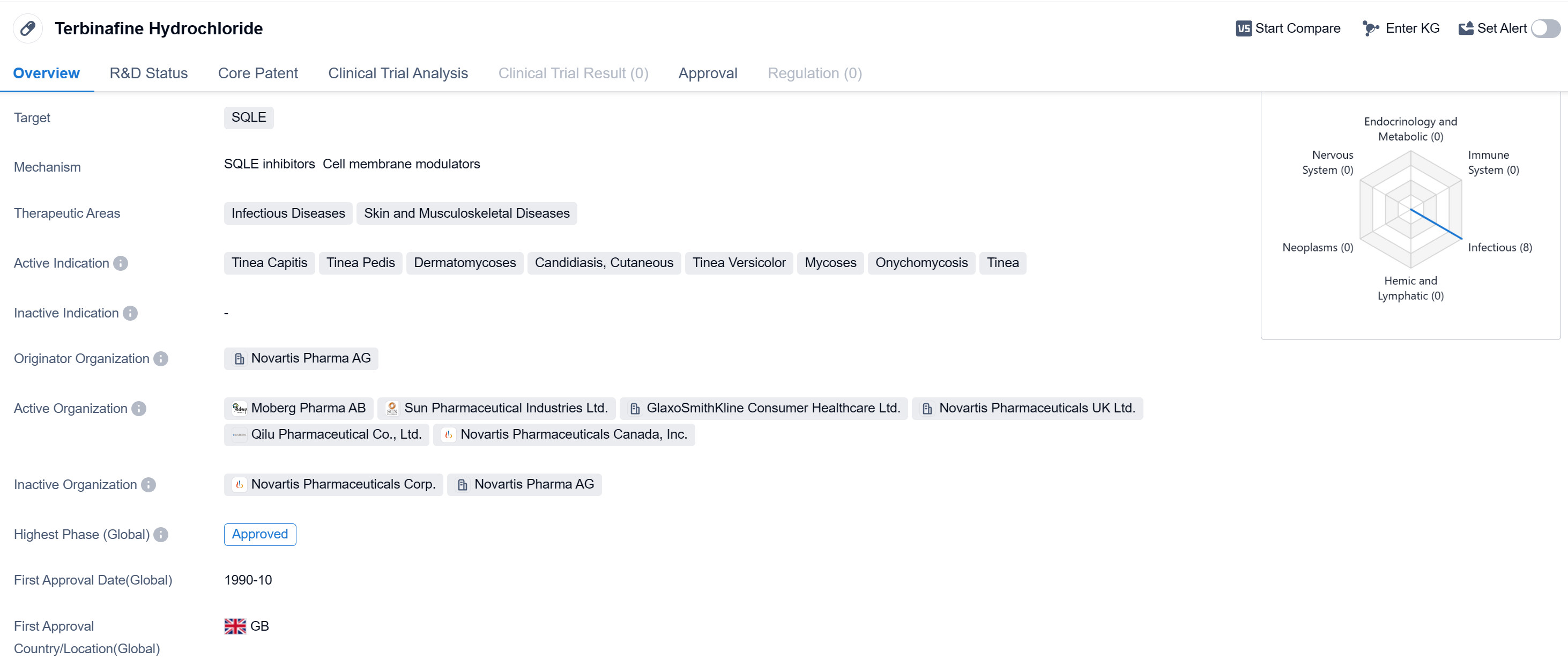
Terbinafine Hydrochloride is a small molecule drug that is primarily used in the treatment of various infectious diseases and skin and musculoskeletal diseases. It targets the enzyme squalene epoxidase (SQLE) to inhibit the synthesis of ergosterol, an essential component of fungal cell membranes.
The drug has been approved for use in multiple indications, including Tinea Capitis (scalp ringworm), Tinea Pedis (athlete's foot), Dermatomycoses (fungal infections of the skin), Candidiasis, Cutaneous (skin yeast infection), Tinea Versicolor (yeast infection of the skin), Mycoses (fungal infections), Onychomycosis (fungal infection of the nails), and Tinea (ringworm).
Terbinafine Hydrochloride was developed by Novartis Pharma AG, a leading pharmaceutical company. It received its first approval in the United Kingdom in October 1990, making it a well-established drug in the market. The drug has also obtained approvals in other countries globally, indicating its widespread use and recognition.
In China, Terbinafine Hydrochloride has also received approval, further expanding its availability and potential patient reach.
As a small molecule drug, Terbinafine Hydrochloride offers several advantages in terms of formulation and delivery. It can be easily synthesized and manufactured, allowing for cost-effective production. Additionally, its small size enables efficient absorption and distribution within the body, enhancing its therapeutic effects.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Terbinafine hydrochloride: SQLE inhibitors and Cell membrane modulators
SQLE inhibitors are a type of drug that target and inhibit the activity of the enzyme SQLE (squalene epoxidase). SQLE is an important enzyme in the cholesterol synthesis pathway, specifically involved in the conversion of squalene to 2,3-oxidosqualene. By inhibiting SQLE, these inhibitors can effectively reduce the production of cholesterol in the body.
From a biomedical perspective, SQLE inhibitors are considered potential therapeutic agents for the treatment of hypercholesterolemia and related cardiovascular diseases. By reducing cholesterol synthesis, these inhibitors can help lower the levels of LDL (low-density lipoprotein) cholesterol, often referred to as "bad" cholesterol, which is a major risk factor for cardiovascular diseases.
Cell membrane modulators, on the other hand, are substances or drugs that can alter the properties or functions of cell membranes. Cell membranes are composed of a lipid bilayer with embedded proteins, and they play a crucial role in maintaining the integrity and functionality of cells.
From a general understanding, cell membrane modulators can have various effects on cell membranes, such as changing their fluidity, permeability, or receptor interactions. These modulators can be used in different fields, including drug delivery systems, where they can enhance the uptake of drugs by cells, or in research settings to study cell membrane dynamics and signaling processes.
In summary, SQLE inhibitors are drugs that target the enzyme involved in cholesterol synthesis, while cell membrane modulators are substances that can alter the properties of cell membranes.
Drug Target R&D Trends for Terbinafine hydrochloride
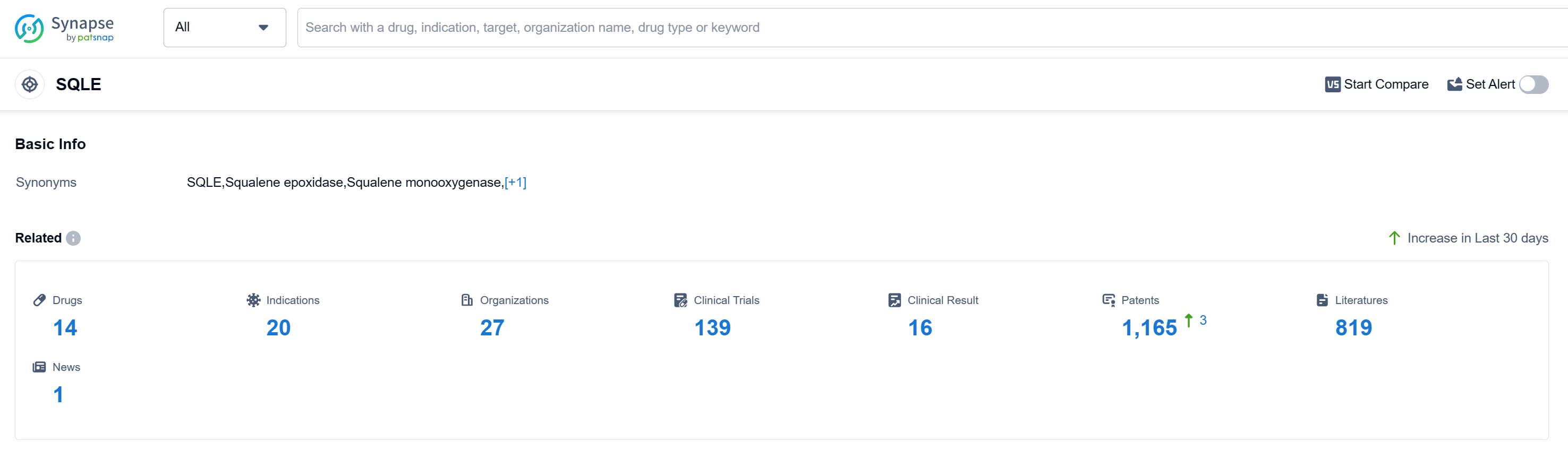
According to Patsnap Synapse, as of 12 Sep 2023, there are a total of 14 SQLE drugs worldwide, from 27 organizations, covering 20 indications, and conducting 139 clinical trials. Based on the analysis of the provided data, the current competitive landscape of target SQLE shows that companies like Novartis AG, Unilever Plc, Viatris Inc., and Pierre Fabre Participations SAS are leading in terms of drug development. The highest stage of development is the Approved phase, with several companies having drugs in this phase. The R&D progress of these companies varies, with drugs in different stages of development. Several drugs under the target SQLE have been approved for indications related to Mycoses, Tinea Pedis, Tinea, Tinea Versicolor, Candidiasis, Cutaneous, tinea cruris, Skin Diseases, Infectious, Onychomycosis, Tinea Capitis, Tinea corporis, Psoriasis, Dermatomycoses, and Vitiligo. Small molecule drugs are progressing rapidly under this target. China, the United States, and Japan are the countries with the most drug development in the Approved phase. China has shown significant progress in drug development for target SQLE.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
Overall, Terbinafine Hydrochloride is a well-established drug in the field of biomedicine, with a proven track record in treating various infectious and skin diseases. Its approval in multiple countries, including China, highlights its global significance and potential for widespread use. As an effective inhibitor of SQLE, Terbinafine Hydrochloride offers a valuable treatment option for patients suffering from fungal infections and related conditions.