Deep Scientific Insights on trimetazidine hydrochloride's R&D Progress
Trimetazidine hydrochloride's R&D Progress
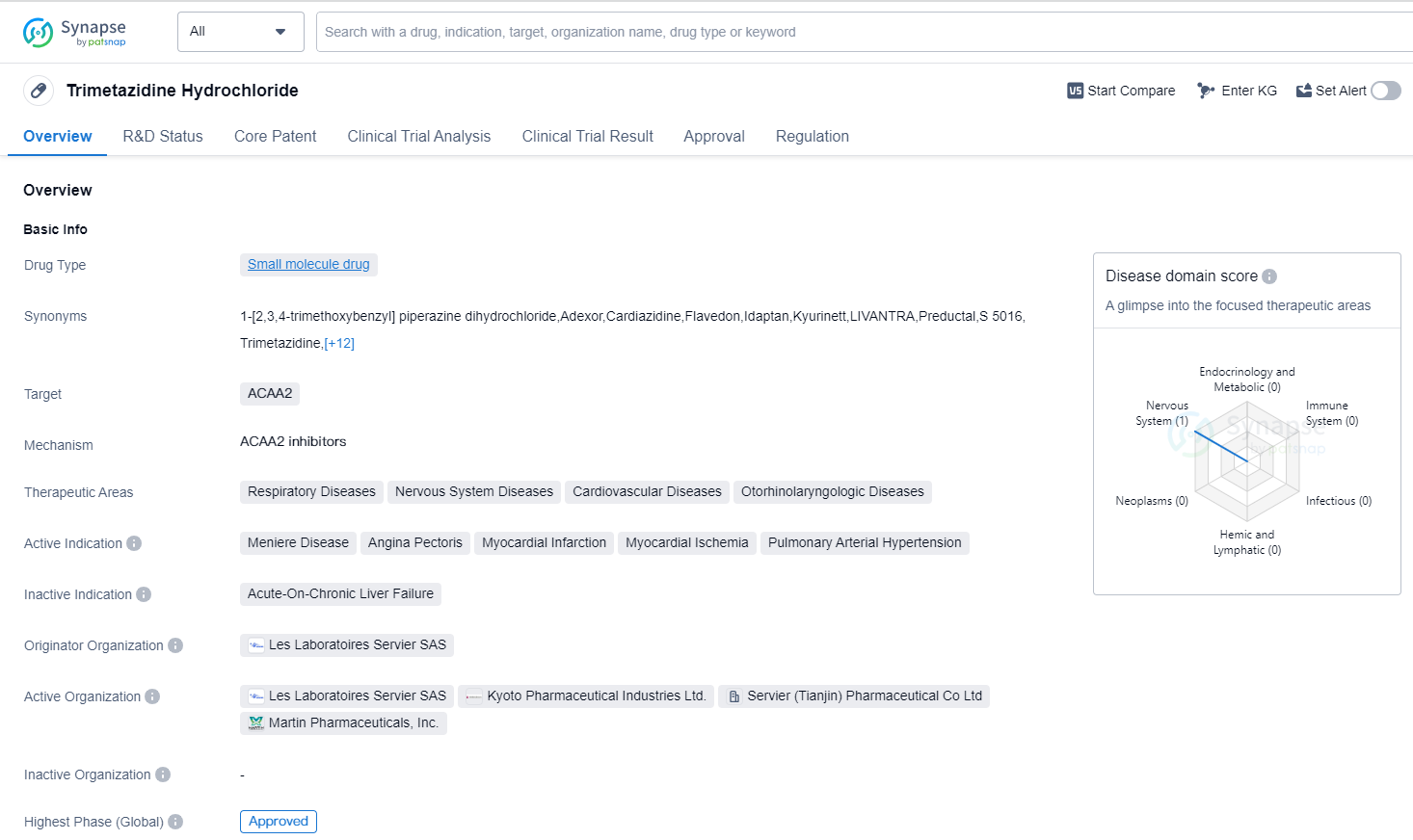
Trimetazidine Hydrochloride is a small molecule drug that targets ACAA2. It is used in the treatment of various therapeutic areas including respiratory diseases, nervous system diseases, cardiovascular diseases, and otorhinolaryngologic diseases. The drug has been approved for the treatment of Meniere Disease, Angina Pectoris, Myocardial Infarction, Myocardial Ischemia, and Pulmonary Arterial Hypertension.
Trimetazidine Hydrochloride was first approved in Japan in January 1968 and is currently approved in multiple countries globally. The drug is developed by Les Laboratoires Servier SAS, an originator organization in the pharmaceutical industry. It has reached the highest phase of development which is approved globally.
In terms of regulatory status, Trimetazidine Hydrochloride has undergone priority review and has also been designated as an orphan drug. Priority review is a regulatory pathway that expedites the review process for drugs that address unmet medical needs or provide significant advancements in treatment. Orphan drug designation is granted to drugs that are intended to treat rare diseases or conditions.
Trimetazidine Hydrochloride's therapeutic applications in respiratory diseases, nervous system diseases, cardiovascular diseases, and otorhinolaryngologic diseases highlight its versatility and potential impact in various medical fields. Its approval for the treatment of Meniere Disease, Angina Pectoris, Myocardial Infarction, Myocardial Ischemia, and Pulmonary Arterial Hypertension further demonstrates its efficacy in addressing critical health conditions.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for trimetazidine hydrochloride: ACAA2 inhibitors
ACAA2 inhibitors are a type of drug that target and inhibit the enzyme acetyl-CoA acyltransferase 2 (ACAA2). This enzyme plays a crucial role in the breakdown of fatty acids in the body. By inhibiting ACAA2, these inhibitors can modulate lipid metabolism and potentially have therapeutic effects in various conditions related to dyslipidemia, such as obesity, metabolic syndrome, and cardiovascular diseases.
From a biomedical perspective, ACAA2 inhibitors can be explored as potential pharmacological interventions to regulate lipid metabolism and address lipid-related disorders. These inhibitors may act by reducing the production of fatty acids or altering their metabolism, leading to a decrease in lipid accumulation and improved metabolic health. However, it is important to note that the development and use of ACAA2 inhibitors are still in the early stages, and further research is needed to determine their safety and efficacy in clinical settings.
Drug Target R&D Trends for trimetazidine hydrochloride
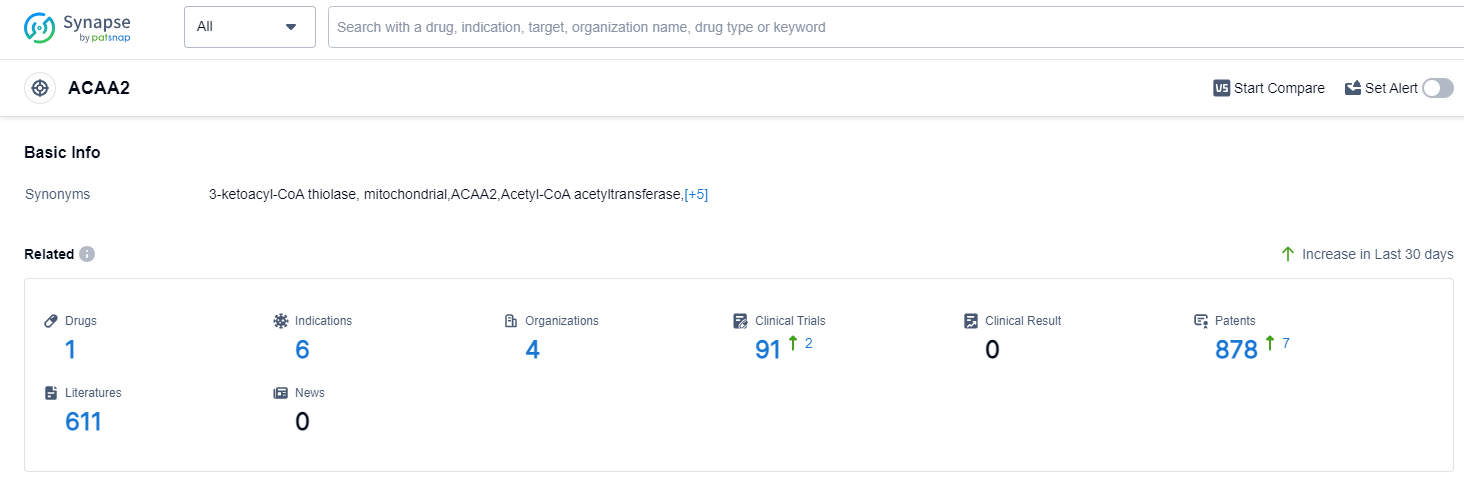
ACAA2, also known as acetyl-CoA acyltransferase 2, plays a crucial role in the human body. It is an enzyme involved in the beta-oxidation of fatty acids, a process that breaks down fatty acids to produce energy. ACAA2 specifically catalyzes the thiolase reaction, which is essential for the breakdown of long-chain fatty acids in mitochondria. This enzyme is primarily found in tissues with high energy demands, such as the heart, liver, and skeletal muscles. ACAA2's role in fatty acid metabolism is vital for maintaining energy balance and ensuring proper functioning of these energy-demanding tissues.
According to Patsnap Synapse, as of 12 Sep 2023, there are a total of 1 ACAA2 drugs worldwide, from 4 organizations, covering 6 indications, and conducting 91 clinical trials.
The analysis of target ACAA2 reveals that Les Laboratoires Servier SAS is the leading company in terms of drug development, with one drug approved and one drug in Phase 3. The approved drugs under target ACAA2 indicate potential for the treatment of various cardiovascular and liver-related conditions. Small molecule drugs are progressing rapidly under this target. The countries/locations developing fastest include China, Japan, and various countries in the European Union. The progress in China, with one drug approved, is noteworthy. Overall, the current competitive landscape for target ACAA2 is promising, with multiple companies and countries actively involved in research and development. The future development of target ACAA2 holds potential for the advancement of treatments for cardiovascular and liver-related conditions.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
Trimetazidine Hydrochloride is a small molecule drug created by Les Laboratoires Servier SAS that targets ACAA2. It has been approved in multiple countries, has priority review status, and has been designated an orphan drug, demonstrating its importance in the pharmaceutical industry. This drug has shown to be effective in treating various diseases, including respiratory, nervous system, cardiovascular, and otorhinolaryngologic diseases, offering hope for patients who suffer from these conditions.