Folinic acid: Detailed Review of its Transformative R&D Success, Mechanism of Action, and Drug Target
Folinic acid's R&D Progress
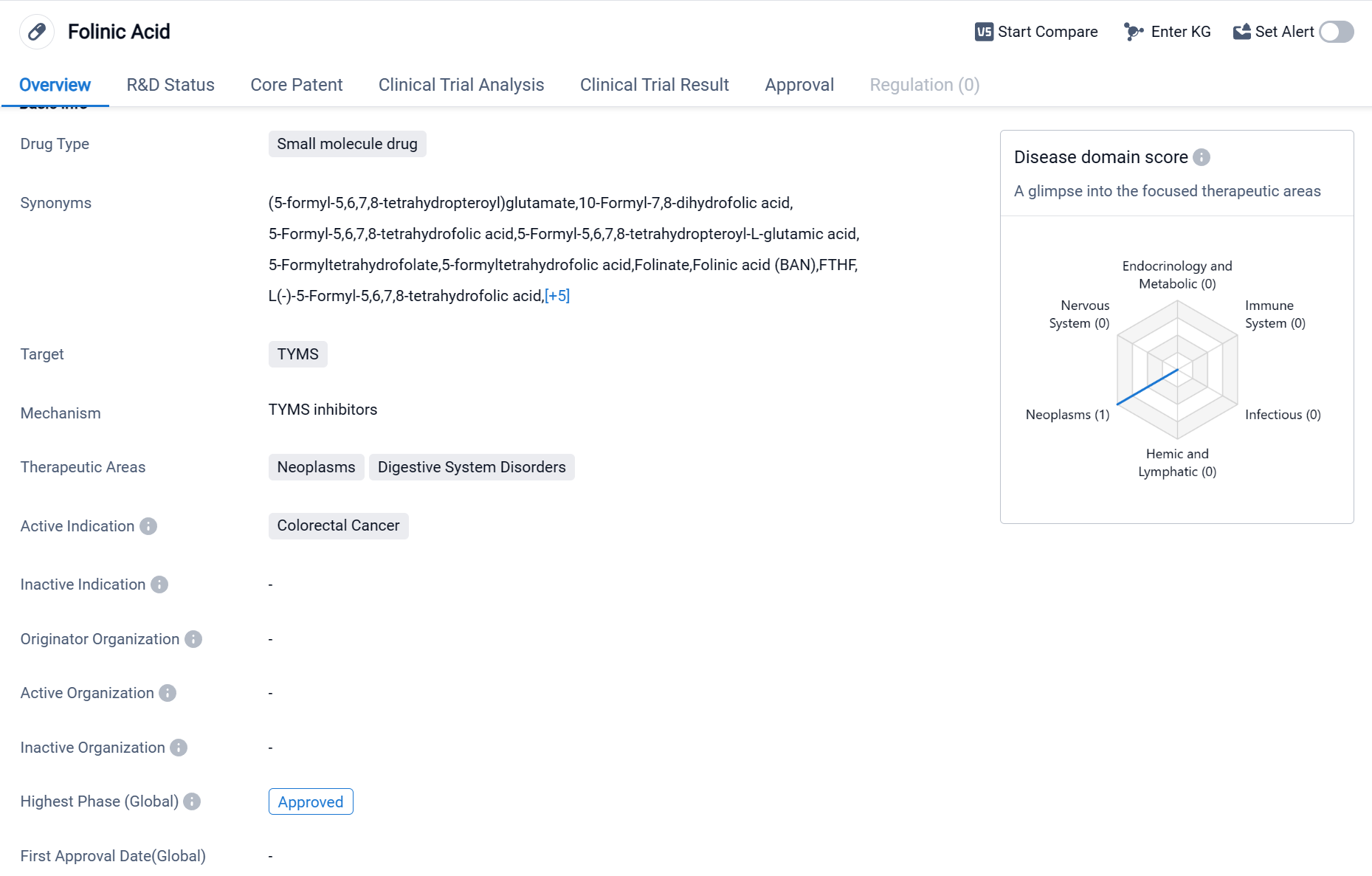
Folinic Acid is a small molecule drug that primarily targets the enzyme thymidylate synthase (TYMS). It is used in the treatment of neoplasms and digestive system disorders, with its active indication being colorectal cancer.
Colorectal cancer is a type of cancer that affects the colon or rectum, and it is one of the most common types of cancer worldwide. Folinic Acid has been approved for use in the treatment of this specific cancer.
As a small molecule drug, Folinic Acid is likely to have a relatively small molecular size and can easily penetrate cell membranes. This characteristic is advantageous as it allows the drug to reach its target, TYMS, and exert its therapeutic effects. TYMS is an enzyme involved in the synthesis of DNA, and inhibiting its activity can disrupt the growth and proliferation of cancer cells.
The therapeutic areas of neoplasms and digestive system disorders encompass a wide range of conditions, indicating that Folinic Acid may have potential applications beyond colorectal cancer. However, without further information, it is difficult to determine the extent of its efficacy in treating other diseases within these therapeutic areas.
The fact that Folinic Acid has reached the highest phase of development, which is approval, suggests that it has undergone extensive clinical trials. This milestone is crucial for pharmaceutical companies as it allows them to market and distribute the drug to patients in need.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Folinic acid: TYMS inhibitors
TYMS inhibitors are a type of medication that work by inhibiting the enzyme thymidylate synthase (TYMS). Thymidylate synthase is an essential enzyme involved in the synthesis of DNA, specifically in the conversion of deoxyuridine monophosphate (dUMP) to deoxythymidine monophosphate (dTMP). By inhibiting TYMS, these inhibitors prevent the production of dTMP, which is necessary for DNA replication and cell division.
From a biomedical perspective, TYMS inhibitors are often used in cancer treatment. Rapidly dividing cancer cells heavily rely on DNA synthesis to grow and proliferate. By inhibiting TYMS and disrupting DNA synthesis, these inhibitors can slow down or halt the growth of cancer cells. This makes TYMS inhibitors a valuable tool in chemotherapy regimens for various types of cancer.
It's important to note that TYMS inhibitors may also affect normal, healthy cells that undergo rapid division, such as cells in the bone marrow and gastrointestinal tract. This can lead to side effects like bone marrow suppression and gastrointestinal disturbances. Therefore, the use of TYMS inhibitors requires careful monitoring and management by healthcare professionals.
Drug Target R&D Trends for Folinic acid
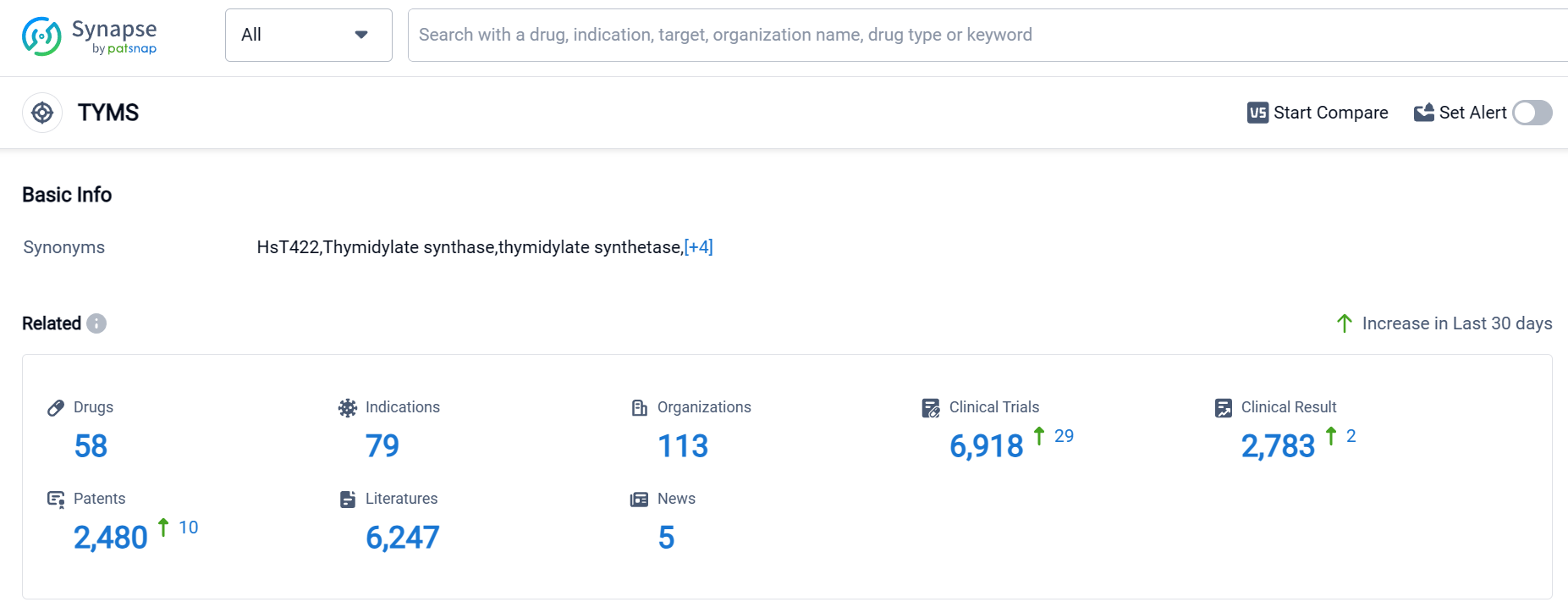
According to Patsnap Synapse, as of 17 Sep 2023, there are a total of 58 TYMS drugs worldwide, from 113 organizations, covering 79 indications, and conducting 6918 clinical trials.
Based on the analysis of the data provided, the current competitive landscape of target TYMS in the pharmaceutical industry is characterized by the growth of companies such as Otsuka Holdings Co., Ltd., Pfizer Inc., and Roche Holding AG. These companies have multiple drugs in various phases of development, indicating their commitment to R&D in this target area.
The most approved indications for drugs targeting TYMS are Colorectal Cancer, Stomach Cancer, Breast Cancer, and Pancreatic Cancer. These indications have the highest number of approved drugs, indicating their relevance and the focus of research in these areas.
Small molecule drugs are progressing most rapidly under the target TYMS, with intense competition indicated by the number of drugs in the approved, preclinical, and inactive phases. Other drug types such as small interfering RNA, oncolytic virus, biological products, drug conjugates, unknown, and enzyme also show potential for future growth and innovation.
China is developing fastest under the target TYMS, with the highest number of approved drugs. Other countries/locations such as Japan, European Union, United States, United Kingdom, and Canada also have significant development in this target area.
Overall, the analysis of the current competitive landscape and future development of target TYMS indicates a growing focus on specific indications, intense competition in small molecule drugs, and the involvement of various countries/locations in the development of drugs targeting TYMS.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Folinic Acid is a small molecule drug that targets TYMS and is primarily used in the treatment of colorectal cancer. Its approval indicates that it has undergone successful clinical trials. While it may have potential applications in other neoplasms and digestive system disorders, further research is needed to determine its efficacy in these areas.