Deep Scientific Insights on Margetuximab's R&D Progress, Mechanism of Action, and Drug Target
Margetuximab's R&D Progress
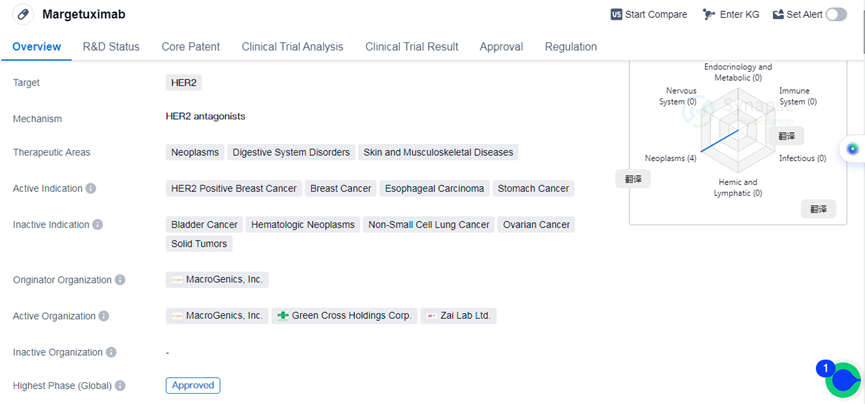
Margetuximab is a monoclonal antibody drug developed by MacroGenics, Inc. It falls under the category of biomedicine and is primarily used for the treatment of neoplasms, digestive system disorders, and skin and musculoskeletal diseases. The drug specifically targets HER2, a protein that is overexpressed in certain types of cancers.
The active indications for Margetuximab include HER2 positive breast cancer, breast cancer, esophageal carcinoma, and stomach cancer.
Margetuximab has received approval for use in the global market and has reached its highest phase of development. Its first approval was granted in December 2020 in the United States.
In terms of regulation, Margetuximab has been granted fast track designation and orphan drug status. Fast track designation is given to drugs that have the potential to address unmet medical needs and allows for expedited development and review processes. Orphan Drug status is granted to drugs that are intended to treat rare diseases or conditions.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Margetuximab: HER2 antagonists
From a biomedical perspective, HER2 antagonists refer to a class of drugs that specifically target the human epidermal growth factor receptor 2 (HER2). HER2 is a protein that overexpressed in certain types of cancer, particularly breast cancer. HER2 antagonists work by binding to the HER2 receptor and inhibiting its signaling pathway, which helps to slow down the growth and spread of cancer cells.
These antagonists can be monoclonal antibodies or small molecule inhibitors. Monoclonal antibodies, such as trastuzumab and pertuzumab, are designed to bind to the HER2 receptor on the surface of cancer cells, blocking its activity and promoting immune-mediated destruction of the cancer cells. Small molecule inhibitors, such as lapatinib and neratinib, are able to penetrate the cell membrane and inhibit the intracellular signaling pathways associated with HER2.
HER2 antagonists are commonly used in the treatment of HER2-positive breast cancer, which is a subtype of breast cancer characterized by overexpression of the HER2 receptor. They can be used alone or in combination with other chemotherapy drugs or targeted therapies to improve treatment outcomes. These drugs have revolutionized the management of HER2-positive breast cancer and have significantly improved patient survival rates.
Drug Target R&D Trends for Margetuximab
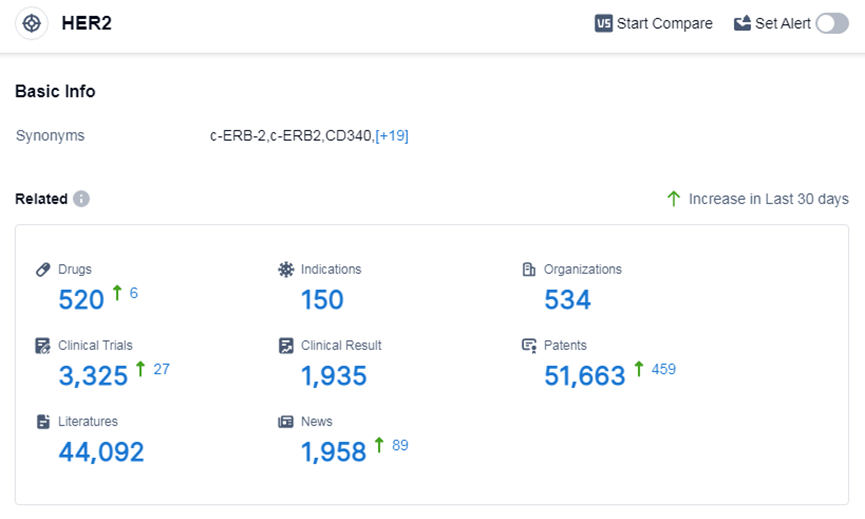
According to Patsnap Synapse, as of 7 Sep 2023, there are a total of 520 HER2 drugs worldwide, from 534 organizations, covering 150 indications, and conducting 3325 clinical trials.
The analysis of the current competitive landscape of target HER2 reveals that Roche Holding AG is the leading company in terms of R&D progress, with drugs in various stages of development. Several drugs have been approved for indications such as breast cancer, HER2 positive breast cancer, and HER2-positive gastric cancer. Monoclonal antibodies and biosimilars are the most rapidly progressing drug types, indicating intense competition. The United States and China develop fastest and have obtained significant progress. The future development of target HER2 is expected to continue with advancements in R&D and the introduction of innovative drugs.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Margetuximab is a monoclonal antibody drug developed by MacroGenics, Inc. It targets HER2 and is primarily used for the treatment of neoplasms, digestive system disorders, and skin and musculoskeletal diseases. The drug has been approved for use in the treatment of HER2 positive breast cancer, breast cancer, esophageal carcinoma, and stomach cancer. It received its first approval in the United States in December 2020 and has been granted fast track and orphan drug designations.




