Temsirolimus: Detailed Review of its Transformative R&D Success, Mechanism of Action, and Drug Target
Temsirolimus's R&D Progress
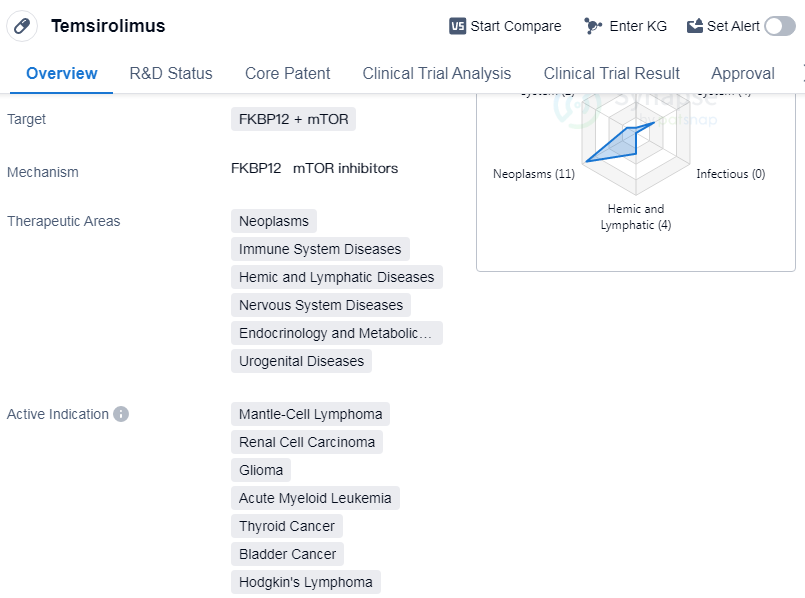
Temsirolimus is a small molecule drug that targets FKBP12 and mTOR. It has been approved for use in neoplasms, immune system diseases, hemic and lymphatic diseases, nervous system diseases, endocrinology and metabolic diseases, and urogenital diseases. The drug has shown efficacy in treating mantle-cell lymphoma, renal cell carcinoma, glioma, acute myeloid leukemia, thyroid cancer, etc.
Temsirolimus was developed by Pfizer Inc., a renowned pharmaceutical company. It received its first approval in the United States in May 2007. The drug has reached the highest phase of development globally, which is the approved stage. In China, it is currently in phase 3 of development.
One notable aspect of Temsirolimus is its orphan drug status. Orphan drugs are medications developed to treat rare diseases or conditions that affect a small number of patients.
Temsirolimus's approval and its diverse therapeutic areas indicate its potential to address various medical needs. Its efficacy in treating different types of cancers, including lymphomas, carcinomas, and gliomas, suggests its broad applicability in oncology. Additionally, its potential in treating diseases related to the immune system, blood, nervous system, and endocrine system highlights its versatility.
As a small molecule drug, Temsirolimus likely possesses characteristics that make it suitable for oral administration, allowing for convenient patient use.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Temsirolimus: FKBP12mTOR inhibitors
FKBP12mTOR inhibitors are a type of drug that target the mammalian target of rapamycin (mTOR) pathway by binding to the FKBP12 protein. From a biomedical perspective, mTOR is a key regulator of cell growth, proliferation, and survival. It plays a crucial role in various cellular processes, including protein synthesis, autophagy, and metabolism. Dysregulation of the mTOR pathway has been implicated in several diseases, including cancer, autoimmune disorders, and neurodegenerative diseases.
FKBP12 is a protein that forms a complex with mTOR and acts as a regulatory subunit. When FKBP12 binds to mTOR, it inhibits its activity. FKBP12mTOR inhibitors, such as rapamycin and its analogs, disrupt the FKBP12-mTOR complex, leading to the inhibition of mTOR signaling. By blocking mTOR, these inhibitors can modulate cell growth and proliferation, suppress immune responses, and induce autophagy.
In the context of biomedicine, FKBP12mTOR inhibitors have shown promising therapeutic potential in various diseases. Additionally, these inhibitors have demonstrated efficacy in the treatment of certain types of cancer, including renal cell carcinoma and mantle cell lymphoma. They are also being investigated for their potential in treating other conditions such as tuberous sclerosis complex, epilepsy, and metabolic disorders.
Overall, FKBP12mTOR inhibitors are a class of drugs that target the mTOR pathway by disrupting the interaction between FKBP12 and mTOR.
Drug Target R&D Trends for Temsirolimus
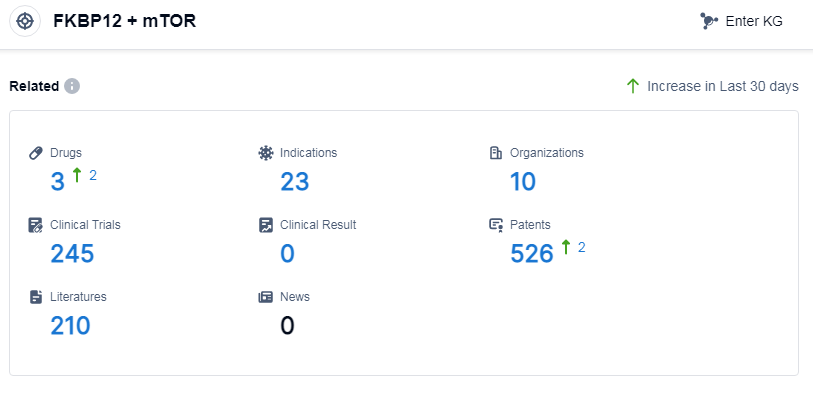
According to Patsnap Synapse, as of 10 Sep 2023, there are a total of 3 FKBP12 and mTOR drugs worldwide, from 10 organizations, covering 23 indications, and conducting 245 clinical trials.
The analysis of the target FKBP12 and mTOR reveals a competitive landscape with Pfizer Inc. being the most prominent company involved in its development. Indications such as mantle-cell lymphoma and renal cell carcinoma have already received approval for drugs targeting this target. Small molecule drugs and chemical drugs are the primary types of drugs being developed. The United States, European Union, Japan, and China are the leading countries/locations in the development of drugs targeting FKBP12 and mTOR.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Temsirolimus is a small molecule drug developed by Pfizer Inc. It targets FKBP12 and mTOR and has been approved for use in various therapeutic areas, including neoplasms and immune system diseases. Its orphan drug status and broad range of active indications highlight its potential to address unmet medical needs. With its approval in the United States and ongoing development in China, Temsirolimus has the potential to make a significant impact in the field of biomedicine.