Perphenazine: Detailed Review of its Transformative R&D Success, Mechanism of Action, and Drug Target
Perphenazine's R&D Progress
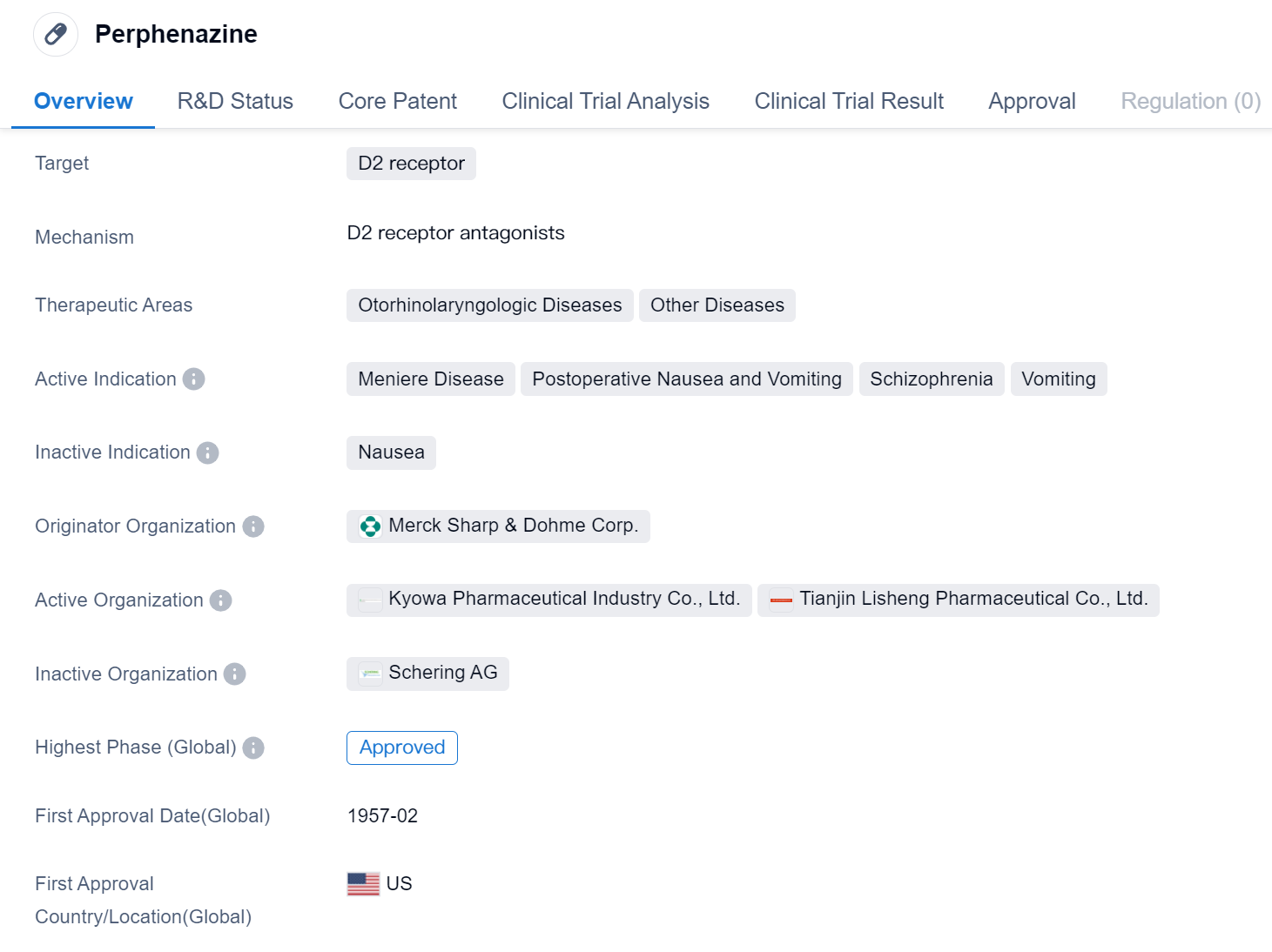
Perphenazine is a small molecule drug that primarily targets the D2 receptor. It has been approved for use in various therapeutic areas, including Otorhinolaryngologic Diseases and Other Diseases. The drug is indicated for the treatment of Meniere Disease, Postoperative Nausea and Vomiting, Schizophrenia, and Vomiting.
Perphenazine was developed by Merck Sharp & Dohme Corp., a renowned pharmaceutical organization. It received its first approval in the United States in February 1957, making it a well-established drug in the market.
As a small molecule drug, Perphenazine is designed to interact with the D2 receptor, which is involved in various physiological processes. By targeting this receptor, the drug aims to modulate its activity and provide therapeutic benefits for patients suffering from different conditions.
The approved therapeutic areas for Perphenazine highlight its potential in treating Otorhinolaryngologic Diseases, which are related to the ear, nose, and throat, as well as Other Diseases. This suggests that the drug may have a broad range of applications beyond its primary indications.
Meniere Disease, characterized by symptoms such as vertigo, hearing loss, and tinnitus, is one of the active indications for Perphenazine. The drug may help alleviate these symptoms and improve the quality of life for patients with this condition.
Postoperative Nausea and Vomiting, a common occurrence after surgical procedures, is another indication for Perphenazine. By targeting the D2 receptor, the drug may help reduce nausea and vomiting in patients recovering from surgery.
Schizophrenia, a chronic mental disorder, is also listed as an active indication for Perphenazine. The drug may be used as part of a comprehensive treatment plan to manage the symptoms associated with this condition.
Vomiting, a symptom that can arise from various underlying causes, is the final active indication for Perphenazine. The drug may be prescribed to control and alleviate vomiting in patients experiencing this symptom.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Perphenazine: D2 receptor antagonists
D2 receptor antagonists are a class of drugs that block or inhibit the activity of D2 receptors in the brain. D2 receptors are a subtype of dopamine receptors, which are involved in various neurological functions, including movement, cognition, emotion, and reward. By blocking D2 receptors, D2 receptor antagonists can modulate dopamine signaling in the brain.
From a biomedical perspective, D2 receptor antagonists are commonly used in the treatment of psychiatric disorders such as schizophrenia and bipolar disorder. By inhibiting the activity of D2 receptors, these drugs can help reduce the symptoms associated with these conditions, such as hallucinations, delusions, and mood disturbances. Additionally, D2 receptor antagonists are also used as antiemetic agents to prevent nausea and vomiting caused by chemotherapy or certain medications.
It's important to note that D2 receptor antagonists may have side effects, including extrapyramidal symptoms (such as tremors and muscle stiffness), sedation, weight gain, and hormonal imbalances. The specific D2 receptor antagonist prescribed and its dosage will depend on the individual's condition and response to the medication. As with any medication, it is essential to follow the prescribed dosage and consult with a healthcare professional for proper guidance and monitoring.
Drug Target R&D Trends for Perphenazine
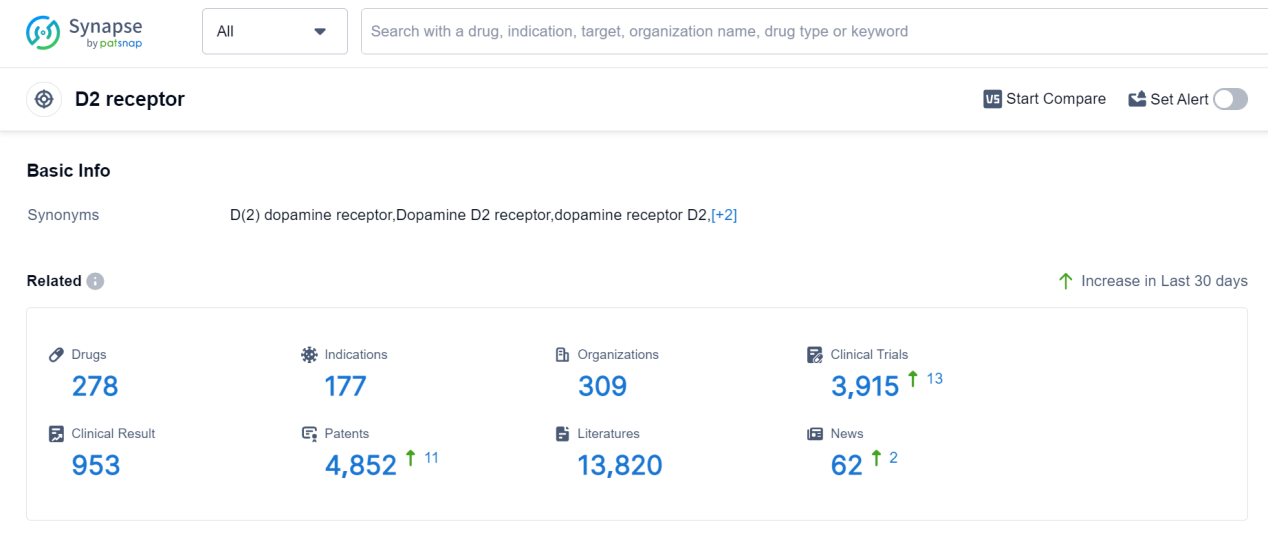
The D2 receptor is a type of dopamine receptor found in the human body. It plays a crucial role in various physiological and neurological processes. As a G-protein coupled receptor, the D2 receptor is involved in the regulation of dopamine neurotransmission, which affects mood, motivation, and reward. Dysfunction of the D2 receptor has been implicated in several psychiatric disorders, including schizophrenia and addiction. Additionally, the D2 receptor is a target for antipsychotic medications, which work by blocking its activity to alleviate symptoms associated with psychosis. Understanding the role of the D2 receptor is essential for developing therapeutic interventions for psychiatric and neurological conditions.
According to Patsnap Synapse, as of 9 Sep 2023, there are a total of 278 D2 receptor drugs worldwide, from 309 organizations, covering 177 indications, and conducting 3915 clinical trials.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
Overall, Perphenazine is a small molecule drug that targets the D2 receptor and has been approved for use in various therapeutic areas. Its active indications include Meniere Disease, Postoperative Nausea and Vomiting, Schizophrenia, and Vomiting. Developed by Merck Sharp & Dohme Corp., Perphenazine has a long history of use, with its first approval dating back to 1957 in the United States.