Unleashing the Power of Penicillin G Procaine: A Comprehensive Review on R&D Breakthroughs
Penicillin G Procaine's R&D Progress
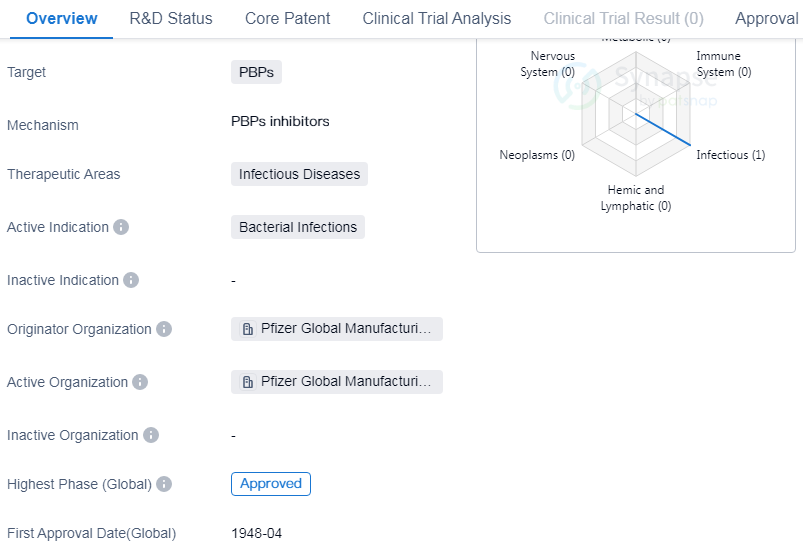
Penicillin G Procaine is a small molecule drug that falls under the therapeutic area of infectious diseases. It is primarily used to treat bacterial infections. The drug targets penicillin-binding proteins (PBPs) to inhibit the synthesis of bacterial cell walls, leading to the destruction of the bacteria.
The originator of Penicillin G Procaine is Pfizer Global Manufacturing China. Pfizer is a renowned pharmaceutical company known for its contributions to the development of various drugs. The drug has reached the highest phase of development, which is approval, indicating that it has successfully undergone rigorous testing and evaluation to ensure its safety and efficacy.
Penicillin G Procaine received its first approval in the United States in April 1948. As a small molecule drug, the drug is likely to have a well-defined chemical structure, making it easier to manufacture and administer.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Penicillin G Procaine: PBPs inhibitors
PBPs inhibitors are a type of drugs that target and inhibit the activity of penicillin-binding proteins (PBPs). PBPs are enzymes found in the cell wall of bacteria and are involved in the synthesis and maintenance of the bacterial cell wall. By inhibiting PBPs, these inhibitors disrupt the formation of the bacterial cell wall, leading to cell lysis and ultimately killing the bacteria.
From a biomedical perspective, PBPs inhibitors are important in the field of antimicrobial therapy. They are commonly used to treat bacterial infections caused by susceptible organisms. By targeting PBPs, these inhibitors effectively inhibit bacterial growth and replication, helping to eliminate the infection. Examples of PBPs inhibitors include penicillin, cephalosporins, and carbapenems, which are widely used antibiotics in clinical practice.
It's worth noting that PBPs inhibitors primarily target bacterial PBPs and are not effective against other types of cells or organisms. They play a crucial role in combating bacterial infections and have significantly contributed to the field of biomedicine.
Drug Target R&D Trends for Penicillin G Procaine
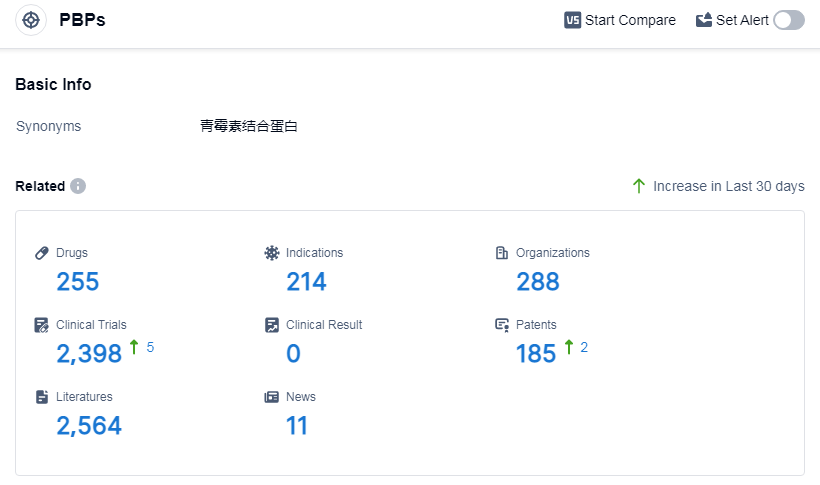
According to Patsnap Synapse, as of 11 Sep 2023, there are a total of 255 PBPs drugs worldwide, from 288 organizations, covering 214 indications, and conducting 2398 clinical trials.
The analysis of target PBPs in the pharmaceutical industry reveals a competitive landscape with several companies actively involved in R&D for this target. Pfizer Inc., Meiji Holdings Co., Ltd., Takeda Pharmaceutical Co., Ltd., Shionogi & Co., Ltd., and Merck & Co., Inc. are the companies growing fastest under the current target. Bacterial infections, infectious diseases, and urinary tract infections are the most common indications for drugs under the target PBPs. Small molecule drugs and synthetic peptides are the drug types progressing most rapidly. China, Japan, and the United States are the countries/locations developing fastest under the current targets, with China showing significant progress. Overall, the analysis suggests a promising future for the development of target PBPs in the pharmaceutical industry.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Penicillin G Procaine is a small molecule drug developed by Pfizer Global Manufacturing China. It is approved for the treatment of bacterial infections and targets PBPs to disrupt bacterial cell wall synthesis. The drug received its first approval in the United States in 1948 and has since been widely used in medical practice. Its approval and originator organization's reputation highlight the drug's credibility and potential impact in the field of biomedicine.