Deutetrabenazine: A Comprehensive Overview of Its Development, Patents, and Therapeutic Applications
Deutetrabenazine is a small molecule drug that targets VMAT2 and is used in the treatment of nervous system diseases, congenital disorders, and other diseases. Its active indications include chorea, tardive dyskinesia, Huntington disease, dystonia, and dystonic disorders. The drug was developed by Teva Pharmaceutical Industries Ltd. and has received approval in the United States in April 2017. Deutetrabenazine has been granted various regulatory designations, such as Overseas New Drugs Urgently Needed in Clinical Settings, Priority Review, Orphan Drug, and Breakthrough Therapy.
The approval of Deutetrabenazine in the United States in 2017 marked a significant milestone for Teva Pharmaceutical Industries Ltd. and the pharmaceutical industry as a whole. With its potential to address unmet medical needs in patients with various movement disorders, it has garnered attention as a promising therapeutic option.
Below, we will use the drug Deutetrabenazine as an example to demonstrate how to quickly obtain information about its chemical structure and patent situation using the Patsnap Chemical.
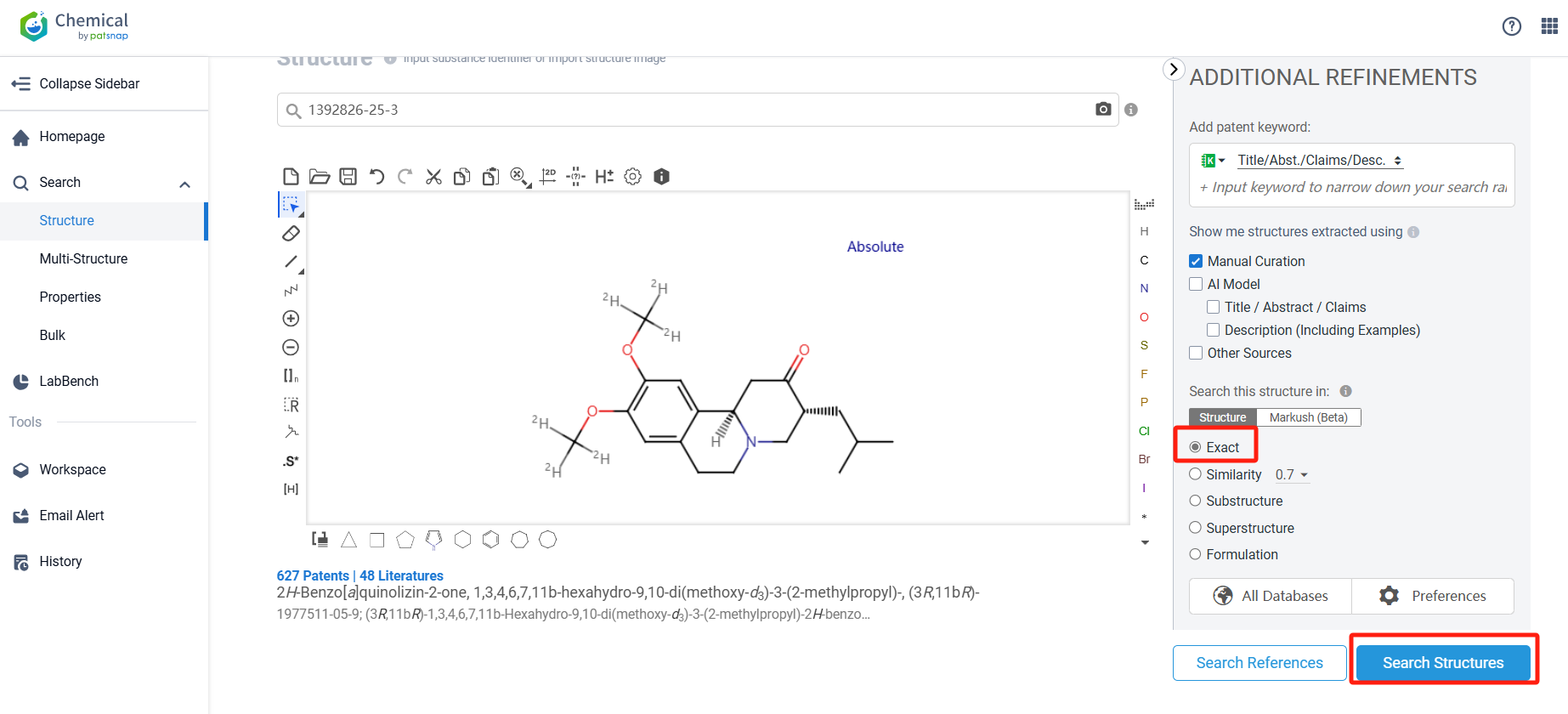
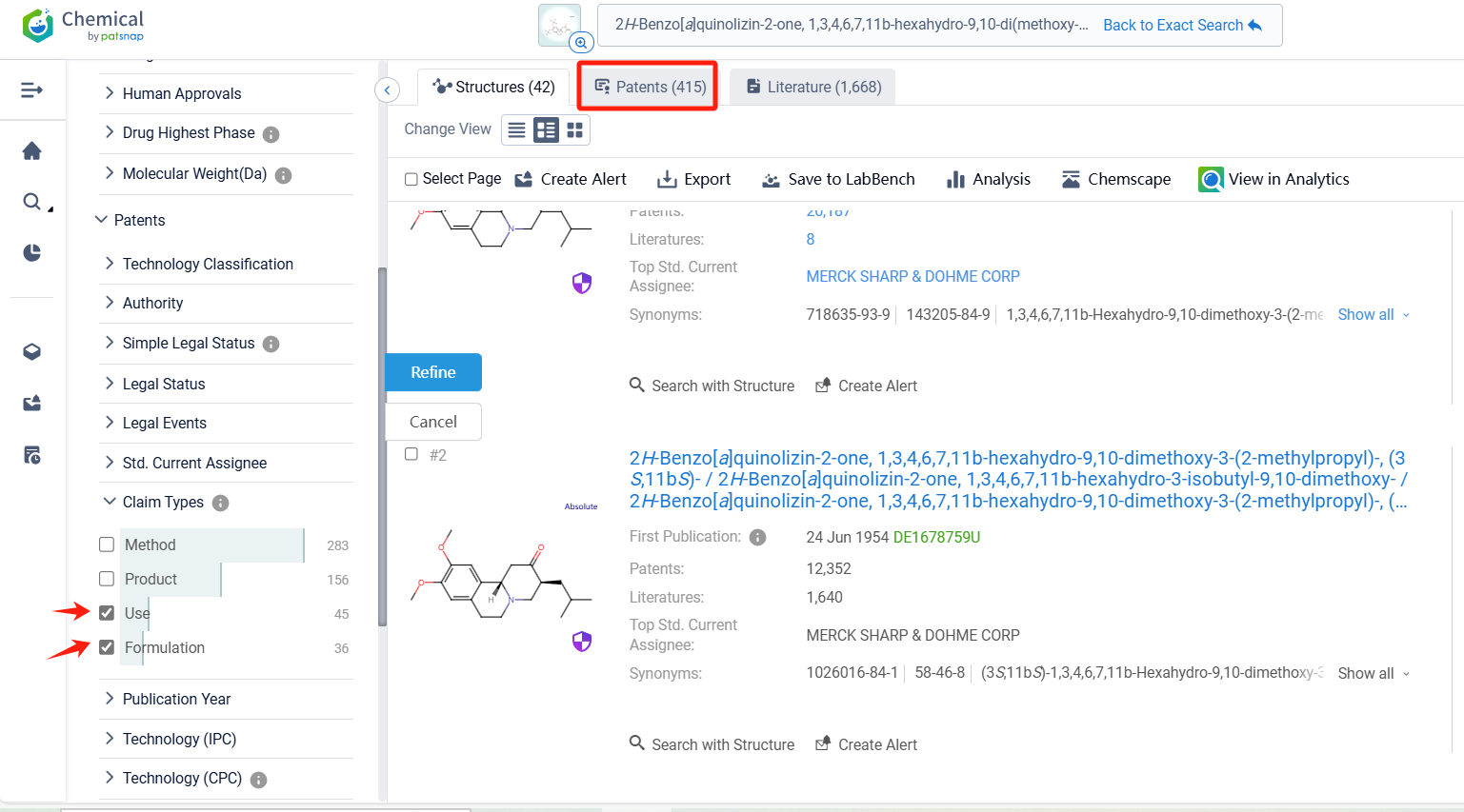
Log in to the Patsnap Chemical. Select the structural search and enter the common identity information of Deutetrabenazine (such as CAS number, generic substance name, molecular formula, SMILES file, etc.). Here, we select "Exact Search", click on search structures, and you can find 415 patents. In the sidebar, select "Formulation" and "Use" under the "Claim Types" to search for patents related to new formulations and new indications. Clicking the "view in Analytics" will direct you to the Patsnap Patent.
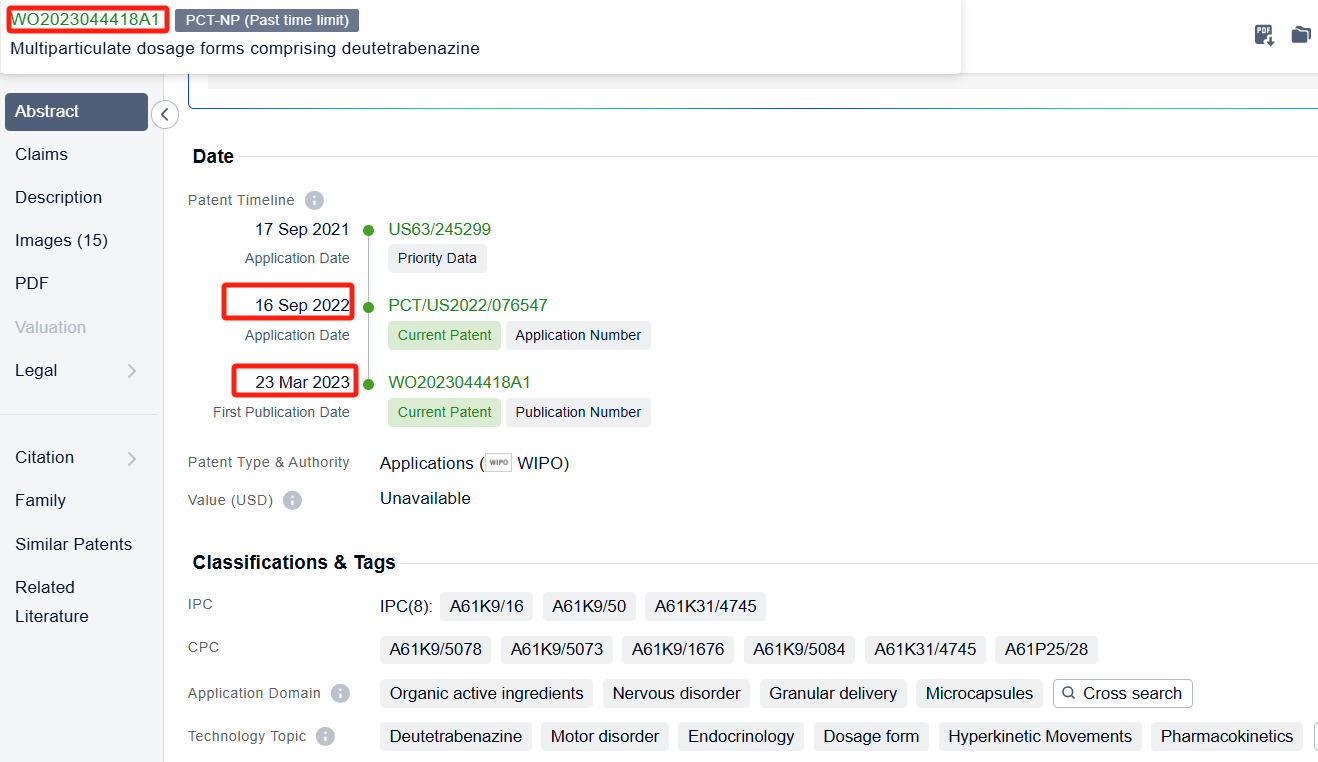
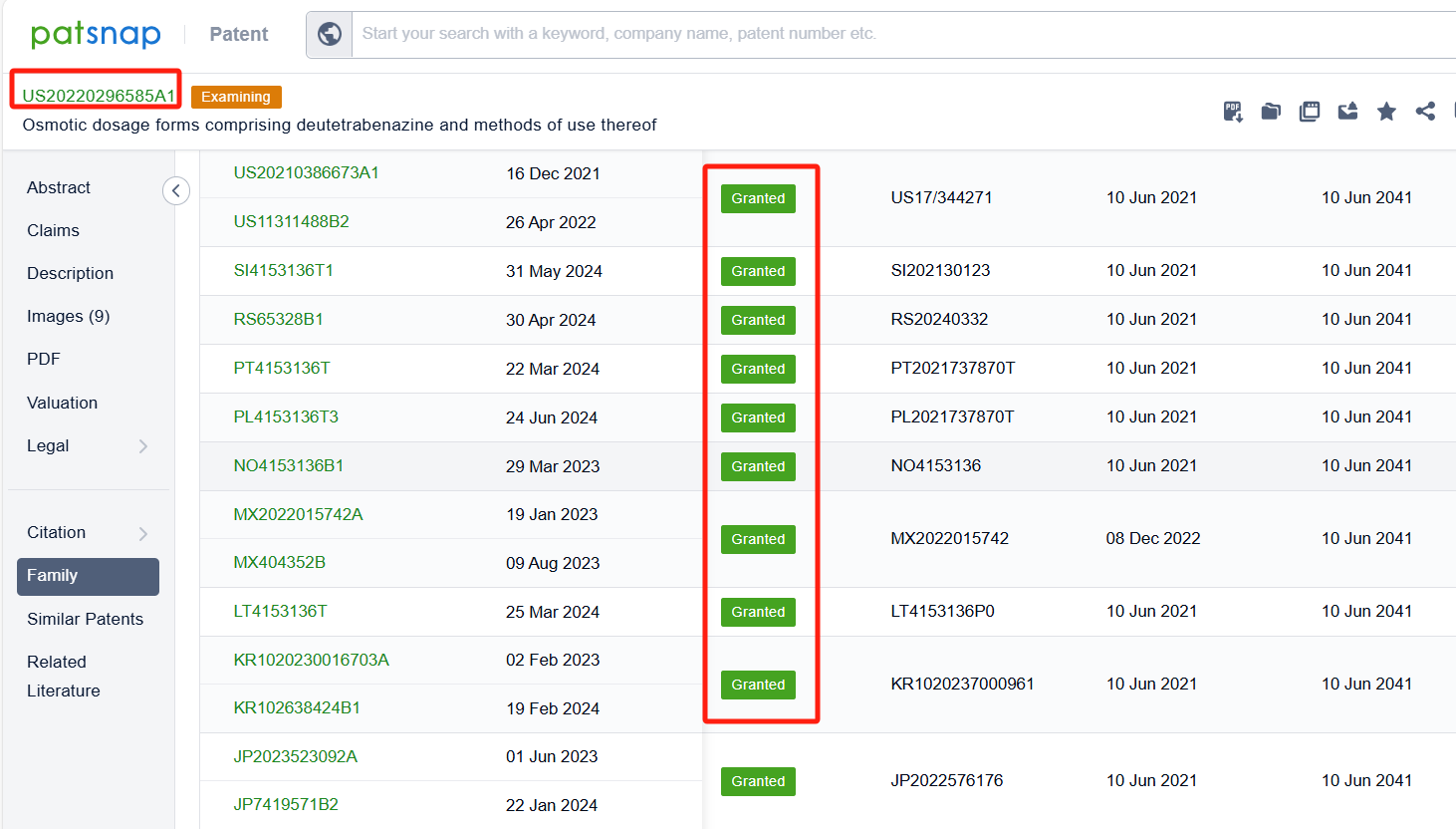
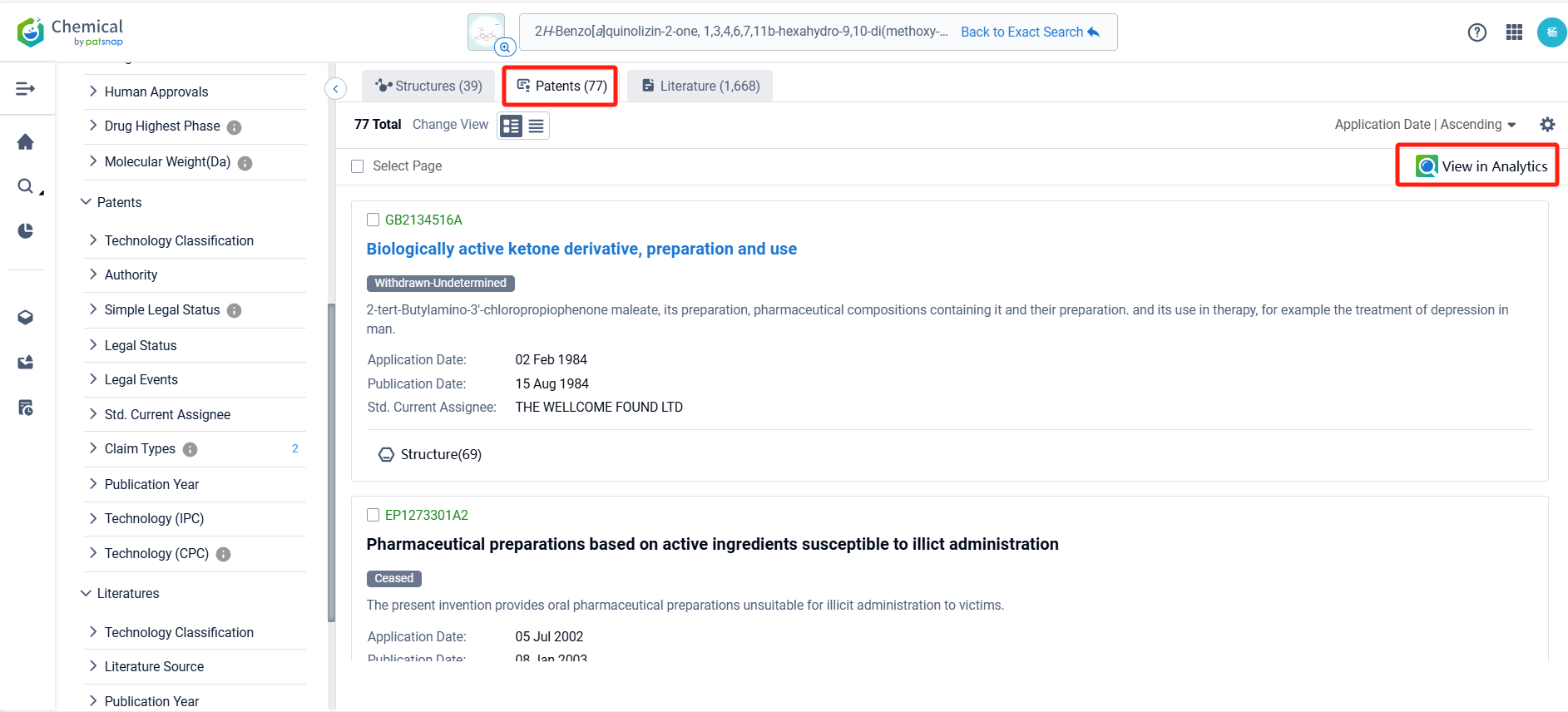 In the Patsnap patent database, we can sort patents by their publication dates to identify the latest patents on Deutetrabenazine. By reviewing the aforementioned patents, we can observe that Auspex Pharmaceuticals, Inc.'s international patent WO2023044418A1(application date 20220916, publication date 20230323) describes a controlled release oral dosage form for twice daily administration of deutetrabenazine. The patent involves making small particles of deutetrabenazine that can be controlled to release the drug at different rates, so that the medication can be taken less frequently but still stay within the therapeutic window. Additionally, Auspex Pharmaceuticals, Inc.'s patent US20220296585A1 (application date 20220608, publication date 20220922) describes a new way to make medication that can be taken once a day. The medication contains a special kind of material called deutetrabenazine, which is made into a tablet with a layer that can be pushed out. The tablet is then surrounded by a layer that allows the medication to be absorbed through the skin. Its corresponding patents in Japan, South Korea, and the United States have all been granted.
In the Patsnap patent database, we can sort patents by their publication dates to identify the latest patents on Deutetrabenazine. By reviewing the aforementioned patents, we can observe that Auspex Pharmaceuticals, Inc.'s international patent WO2023044418A1(application date 20220916, publication date 20230323) describes a controlled release oral dosage form for twice daily administration of deutetrabenazine. The patent involves making small particles of deutetrabenazine that can be controlled to release the drug at different rates, so that the medication can be taken less frequently but still stay within the therapeutic window. Additionally, Auspex Pharmaceuticals, Inc.'s patent US20220296585A1 (application date 20220608, publication date 20220922) describes a new way to make medication that can be taken once a day. The medication contains a special kind of material called deutetrabenazine, which is made into a tablet with a layer that can be pushed out. The tablet is then surrounded by a layer that allows the medication to be absorbed through the skin. Its corresponding patents in Japan, South Korea, and the United States have all been granted.
In summary, Deutetrabenazine is a small molecule drug that targets VMAT2 and has been approved for the treatment of nervous system diseases, congenital disorders, and other diseases. Its active indications include chorea, tardive dyskinesia, Huntington disease, dystonia, and dystonic disorders. First approved in the United States in 2017, this drug has received various regulatory designations, highlighting its potential as a treatment option for patients with movement disorders.
AI built to maximize IP and R&D efficiency
Redefine chemical FTO with a range of structure retrieval options at your fingertips, from exact matches to similarity searches, all powered by deep data processing techniques and proprietary AI algorithms to eliminate the risk of omitting key results.