NextCure Showcases Preclinical Results for LNCB74 and Clinical Biomarker Data for NC410 at SITC Meeting
NextCure, Inc. (Nasdaq: NXTC), a biopharmaceutical company in the clinical stage that focuses on the discovery and development of innovative therapies for cancer, has announced pre-clinical findings related to LNCB74, a B7-H4-targeting antibody-drug conjugate (ADC) developed in collaboration with LigaChem Biosciences (LCB) (KOSDAQ: 141080). Additionally, biomarker information from the NC410 combination study involving pembrolizumab in patients with naïve and refractory microsatellite stable (MSS)/microsatellite instability-low (MSI-L) colorectal cancer (CRC) will be shared at poster sessions during the Society for Immunotherapy of Cancer (SITC) annual meeting.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
“The preclinical findings for LNCB74, our antibody-drug conjugate program targeting B7-H4, continue to highlight its potential to be a leading therapy with distinct advantages over existing B7-H4 ADCs,” stated Michael Richman, the president and CEO of NextCure. “We are on schedule to submit an IND application to the FDA by the end of this year and plan to promptly progress the program into clinical development.”
Preclinical Findings on LNCB74 B7-H4 Antibody Drug Conjugate (ADC)
LNCB74 is an ADC formed by linking a B7-H4 antibody to the microtubule-disrupting agent monomethyl auristatin E (MMAE) with a drug-to-antibody ratio of 4 (DAR4). This ADC utilizes a glucuronidase-cleavable, site-specific linker attached to a modified cysteine in the light chain of the antibody, developed using LigaChem Biosciences’ ConjuAllTM technology. This design enhances circulation stability, enables specific payload release in tumor cells, and minimizes payload release in non-tumor tissues. An Fc mitigating mutation is incorporated into LNCB74 to reduce its binding and uptake by immune cells expressing Fc receptors. The ConjuAll technology’s selective cleavage and tumor cell release, complemented by reduced off-target uptake through modified Fc interactions, aim to enhance the safety and therapeutic efficacy of LNCB74 relative to other ADCs targeting B7-H4.
Key Insights:
- The B7-H4 protein is predominantly expressed in various tumor types, while showing limited expression in normal human tissues, indicating a potentially wide therapeutic index for a B7-H4-directed ADC.
- LNCB74 specifically targets B7-H4 expressing tumor cells and is internalized quickly in a manner dependent on the target.
- LNCB74 exhibits strong cytotoxic effects, with sub-nanomolar to low nanomolar EC50 values across several B7-H4-positive cancer cell lines.
- Promising anti-tumor effects were observed in various CDX and PDX tumor models, where a single dose of 3 mg/kg led to lasting tumor regression, indicating activity that may be equal to or exceed that of other published B7-H4 targeting ADCs.
- LNCB74 has shown favorable pharmacokinetics and stability in rodent models.
- In cynomolgus monkeys, LNCB74 was well tolerated at doses up to 10 mg/kg.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
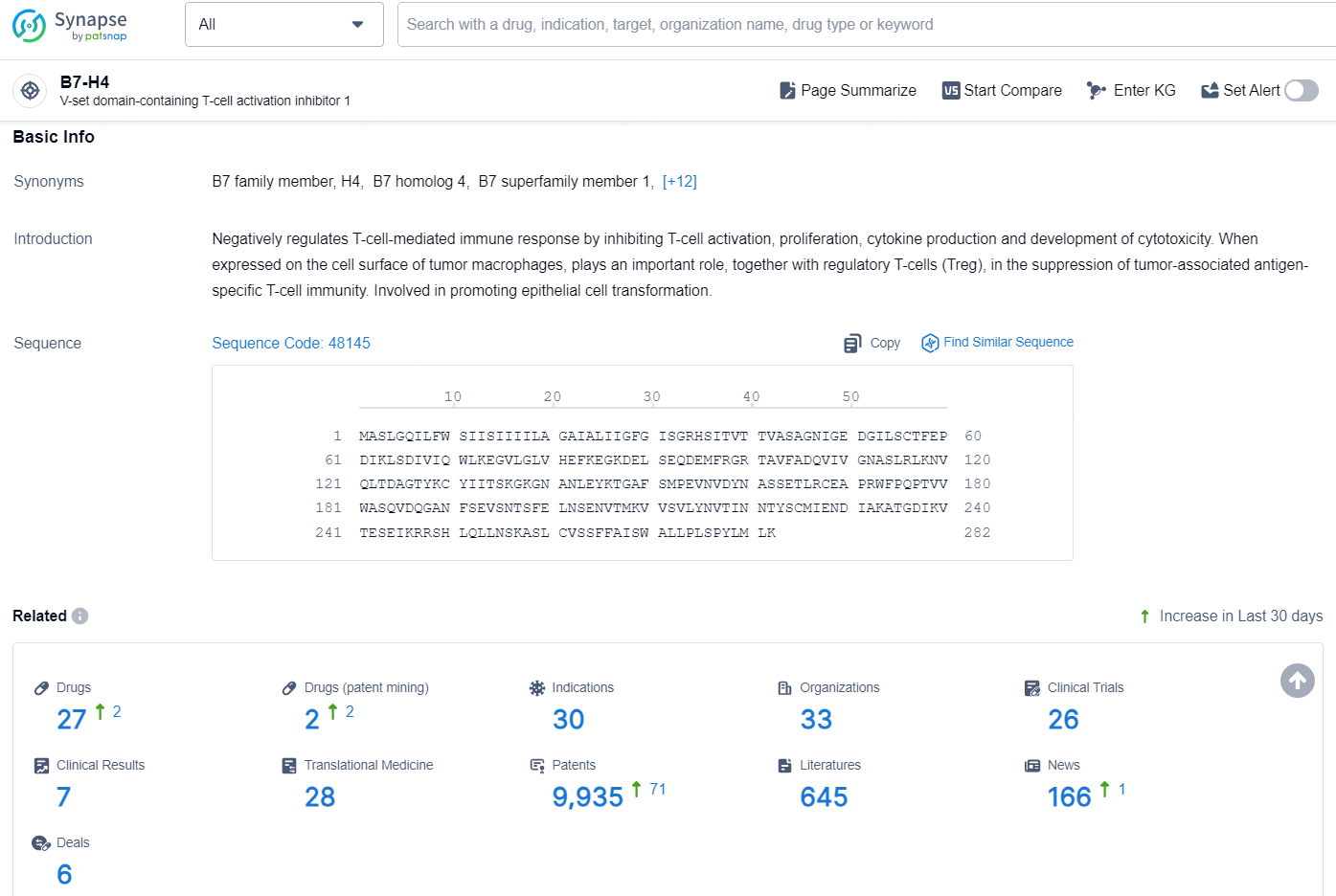
According to the data provided by the Synapse Chemical, As of November 7, 2024, there are 27 investigational drugs for the B7-H4 target, including 30 indications, 33 R&D institutions involved, with related clinical trials reaching 26, and as many as 9935 patents.
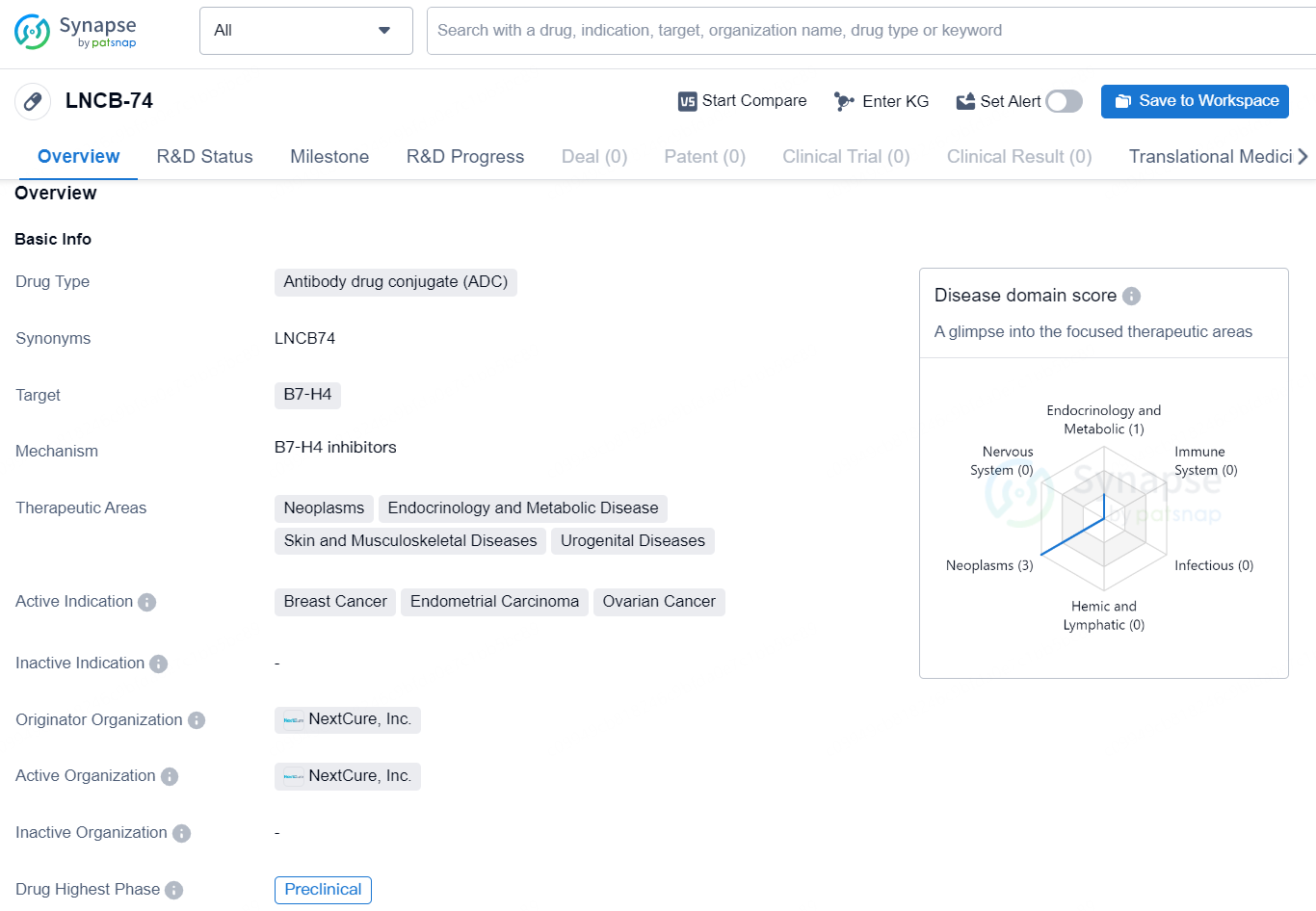
The drug LNCB-74 is an antibody drug conjugate (ADC) that targets B7-H4. It is being developed for the treatment of neoplasms, endocrinology and metabolic diseases, skin and musculoskeletal diseases, and urogenital diseases. The active indications for LNCB-74 include breast cancer, endometrial carcinoma, and ovarian cancer. The originator organization of this drug is NextCure, Inc., and the highest phase of development for LNCB-74 is currently preclinical.