Dexmedetomidine hydrochloride Unveiled: A Detailed Overview of its Revolutionary R&D Breakthroughs
Dexmedetomidine hydrochloride's R&D Progress
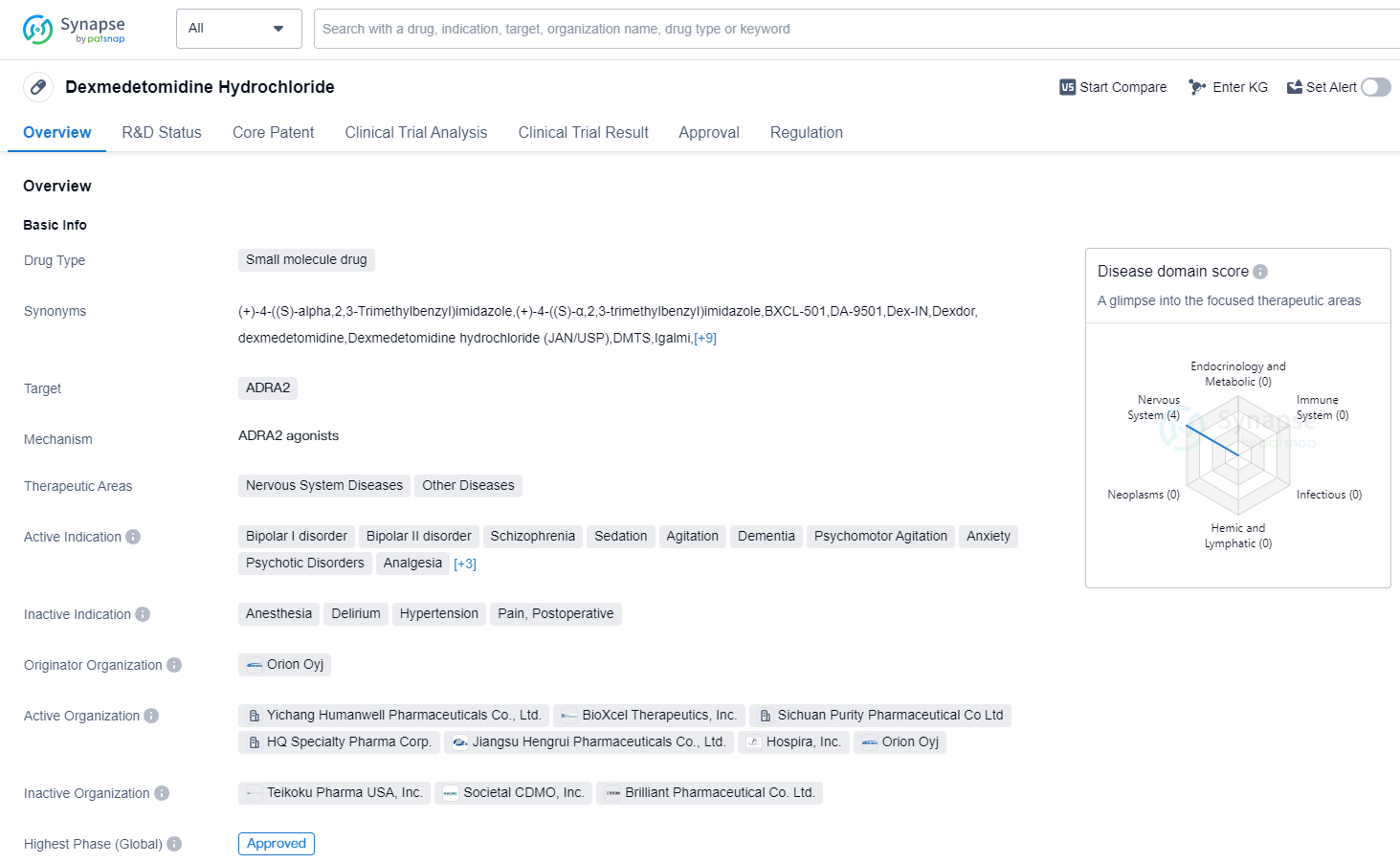
Dexmedetomidine Hydrochloride is a small molecule drug that targets ADRA2 receptors in the body. It is primarily used in the treatment of various nervous system diseases and other related conditions. The drug has been approved for use in the treatment of bipolar I disorder, bipolar II disorder, schizophrenia, sedation, agitation, dementia, psychomotor agitation, anxiety, psychotic disorders, analgesia, substance withdrawal syndrome, opioid-related disorders, and sleep initiation and maintenance disorders.
The originator organization of Dexmedetomidine Hydrochloride is Orion Oyj, a pharmaceutical company. The drug has reached the highest phase of development which is approved globally. It received its first approval in the United States in December 1999.
In terms of regulatory status, Dexmedetomidine Hydrochloride has undergone priority review and fast track processes. These regulatory designations indicate that the drug has demonstrated potential benefits in treating serious or life-threatening conditions and has the potential to address unmet medical needs.
Dexmedetomidine Hydrochloride is an important drug in the field of biomedicine, particularly in the treatment of various nervous system diseases. Its approval for multiple indications highlights its versatility and potential to address a wide range of conditions. The drug's small molecule nature allows for efficient delivery and targeting of ADRA2 receptors, which play a crucial role in the functioning of the nervous system.
The global recognition and potential for widespread use of Dexmedetomidine Hydrochloride is highlighted by its approval in both the United States and China, with its first approval dating back to 1999.s been in use for over two decades, further establishing its safety and efficacy profile.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for dexmedetomidine hydrochloride: ADRA2 agonists
ADRA2 agonists are a type of medication that activate or stimulate the alpha-2 adrenergic receptors in the body. These receptors are found in various tissues and organs, including the brain, blood vessels, and peripheral nervous system. By binding to and activating these receptors, ADRA2 agonists can have different effects depending on the specific receptor subtype and location.
From a biomedical perspective, ADRA2 agonists are commonly used in the treatment of conditions such as hypertension (high blood pressure), anxiety disorders, and attention deficit hyperactivity disorder (ADHD). By activating alpha-2 adrenergic receptors in the brain, these medications can help regulate the release of norepinephrine, a neurotransmitter involved in mood regulation and stress response. This can lead to a decrease in anxiety symptoms and improved focus and attention in individuals with ADHD.
In the context of hypertension, ADRA2 agonists work by reducing the release of norepinephrine from nerve endings, leading to a decrease in sympathetic outflow and subsequent relaxation of blood vessels. This results in lowered blood pressure levels.
It's important to note that ADRA2 agonists can have side effects, including sedation, dry mouth, and dizziness. They should be used under the supervision of a healthcare professional and as prescribed.
Drug Target R&D Trends for dexmedetomidine hydrochloride
ADRA2, or alpha-2 adrenergic receptor, plays a crucial role in the human body. These receptors are found in various tissues and organs, including the central nervous system, cardiovascular system, and gastrointestinal tract. ADRA2 receptors are involved in regulating blood pressure, heart rate, and neurotransmitter release. Activation of these receptors leads to vasoconstriction, reducing blood flow and lowering blood pressure. Additionally, ADRA2 receptors modulate the release of norepinephrine and other neurotransmitters, influencing mood, cognition, and stress response. Understanding the role of ADRA2 receptors is essential for developing drugs that target these receptors to treat conditions such as hypertension, anxiety, and depression.
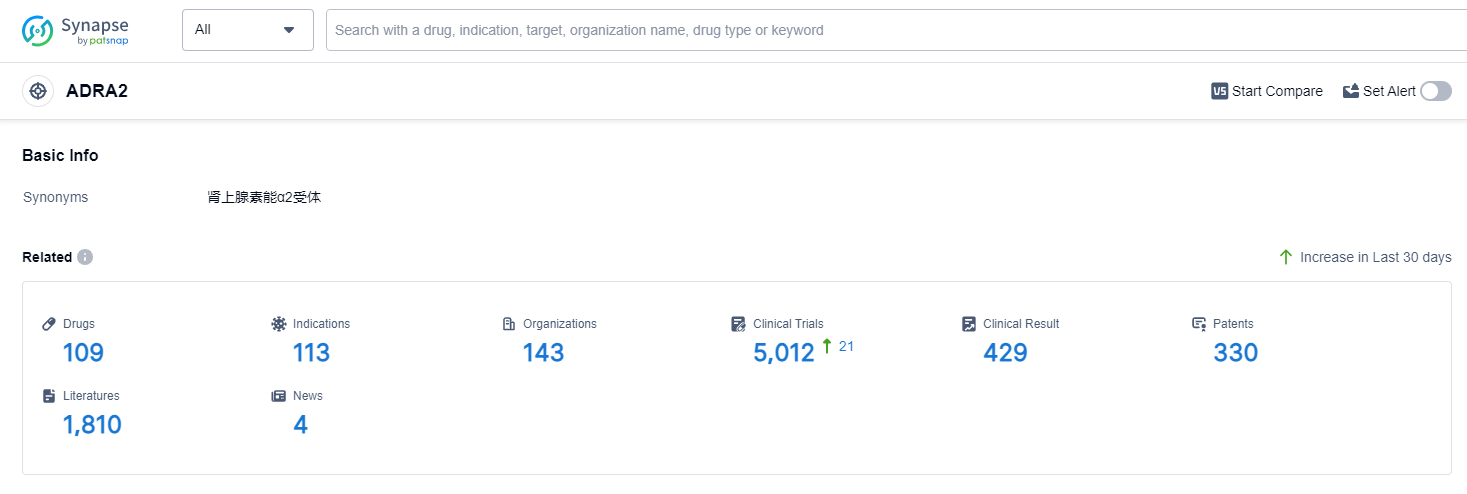
According to Patsnap Synapse, as of 12 Sep 2023, there are a total of 109 ADRA2 drugs worldwide, from 143 organizations, covering 113 indications, and conducting 5012 clinical trials.
The analysis of target ADRA2 reveals a competitive landscape with multiple companies making progress in the development of drugs. Merck & Co., Inc., Senju Pharmaceutical Co., Ltd., AbbVie, Inc., Pfizer Inc., and Organon & Co. are among the companies growing fastest under this target. The indications with the highest number of approved drugs include hypertension, ocular hypertension, glaucoma, and depressive disorder. Small molecule drugs dominate the drug types progressing rapidly under target ADRA2. China, the United States, Japan, the European Union, and the United Kingdom are the countries/locations developing fastest under this target, with China showing significant progress.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
Overall, Dexmedetomidine Hydrochloride is a significant drug in the pharmaceutical industry, with its approval for multiple indications and its regulatory designations reflecting its potential to address various medical needs. Its originator organization, Orion Oyj, has successfully developed and brought this drug to market, contributing to advancements in the treatment of nervous system diseases and other related conditions.