Duvelisib: Detailed Review of its Transformative R&D Success, Mechanism of Action, and Drug Target
Duvelisib's R&D Progress
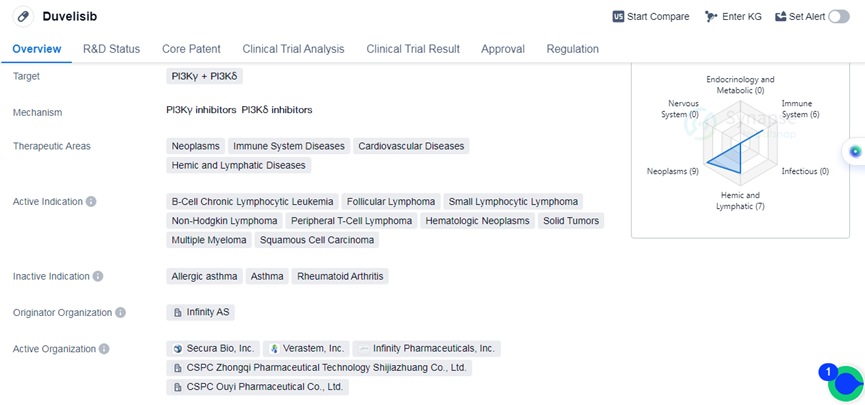
Duvelisib is a small molecule drug that targets PI3Kγ and PI3Kδ. It is primarily used in the treatment of various diseases related to the immune system, cardiovascular system, and hemic and lymphatic systems. The drug has shown efficacy in treating neoplasms, immune system diseases, cardiovascular diseases, and hemic and lymphatic diseases.
Duvelisib has been approved for the treatment of B-cell chronic lymphocytic leukemia, follicular lymphoma, small lymphocytic lymphoma, non-hodgkin lymphoma, peripheral T-cell lymphoma and etc. This wide range of approved indications highlights the potential of Duvelisib in treating various types of cancers and lymphomas.
Duvelisib was developed by Infinity AS, an originator organization specializing in the pharmaceutical industry. It has received approval in the global market, indicating its potential for international use. The drug was first approved in the United States in September 2018, marking its initial entry into the market.
Duvelisib has undergone regulatory processes such as priority review, accelerated approval, fast track, and orphan drug designation. These regulatory designations highlight the importance and urgency of the drug in addressing unmet medical needs and providing potential benefits to patients.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Duvelisib: PI3Kγ inhibitors and PI3Kδ inhibitors
PI3Kγ inhibitors and PI3Kδ inhibitors are two different types of drugs that target specific isoforms of the enzyme phosphoinositide 3-kinase (PI3K).
From a biomedical perspective, PI3K is a crucial enzyme involved in various cellular processes, including cell growth, proliferation, and survival. It plays a significant role in signaling pathways that regulate immune responses, inflammation, and cancer development.
PI3Kγ inhibitors specifically target the gamma isoform of PI3K, while PI3Kδ inhibitors target the delta isoform. By inhibiting these specific isoforms, these drugs can modulate the activity of PI3K and potentially disrupt the signaling pathways associated with specific diseases.
In the context of biomedicine, PI3Kγ inhibitors and PI3Kδ inhibitors are being investigated as potential therapeutic agents for various conditions. For example, PI3Kδ inhibitors have shown promise in the treatment of certain types of blood cancers, such as chronic lymphocytic leukemia (CLL). By selectively inhibiting PI3Kδ, these drugs can interfere with the survival and proliferation of cancer cells, leading to their destruction.
It is important to note that the use of PI3K inhibitors, including PI3Kγ inhibitors and PI3Kδ inhibitors, is still under clinical investigation, and their safety and efficacy profiles are being evaluated in ongoing research and clinical trials.
Drug Target R&D Trends for Duvelisib
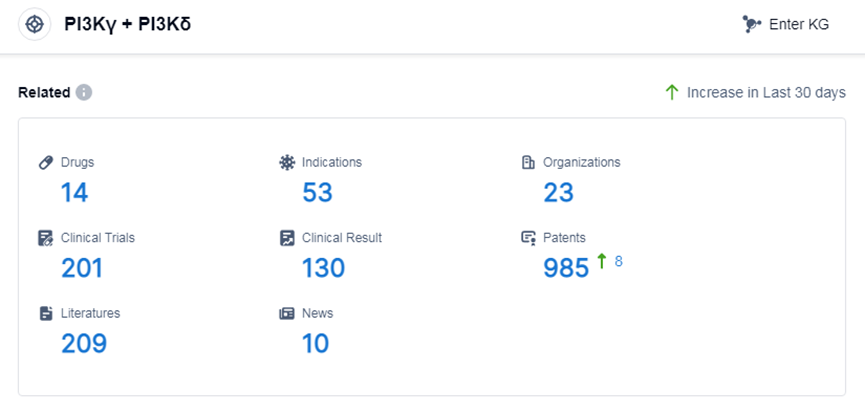
According to Patsnap Synapse, as of 7 Sep 2023, there are a total of 14 PI3Kγ and PI3Kδ drugs worldwide, from 23 organizations, covering 53 indications, and conducting 201 clinical trials.
The analysis of the current competitive landscape of target PI3Kγ and PI3Kδ reveals that multiple companies are actively involved in the development of drugs targeting this pathway. Secura Bio, Inc. leads the way in terms of the highest stage of development, followed by CSPC Pharmaceutical Group Ltd. and Novartis AG. The indications for which drugs have been approved under this target include B-cell chronic lymphocytic leukemia, follicular lymphoma, small lymphocytic lymphoma, and hematologic neoplasms. Small molecule drugs are progressing rapidly, with one approved drug and several in the preclinical and inactive stages. China is one of the countries/locations with the most significant development progress, and it has shown progress in the development of drugs targeting PI3Kγ and PI3Kδ. Overall, the target PI3Kγ and PI3Kδ presents a competitive landscape with multiple companies and potential therapeutic applications in lymphomas and hematologic neoplasms.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Duvelisib is a small molecule drug that targets PI3Kγ and PI3Kδ. It has been approved for the treatment of various neoplasms, immune system diseases, cardiovascular diseases, and hemic and lymphatic diseases. Developed by Infinity AS, Duvelisib has received global approval, with its first approval in the United States in September 2018. The drug has undergone regulatory processes such as priority review, accelerated approval, fast track, and orphan drug designation. These characteristics make Duvelisib a promising drug in the field of biomedicine, offering potential therapeutic options for patients with different types of cancers and lymphomas.