Elevation Oncology Expands Pipeline with HER3-Targeting ADC EO-1022 for Solid Tumors
Elevation Oncology, Inc. (Nasdaq: ELEV), a forward-thinking oncology firm dedicated to identifying and developing targeted cancer treatments for patients suffering from various solid tumors with substantial unmet medical requirements, has officially designated EO-1022 as its candidate for development as a HER3 ADC. EO-1022 is presently advancing through the preclinical stage, and Elevation Oncology anticipates submitting an investigational new drug (IND) application in 2026.
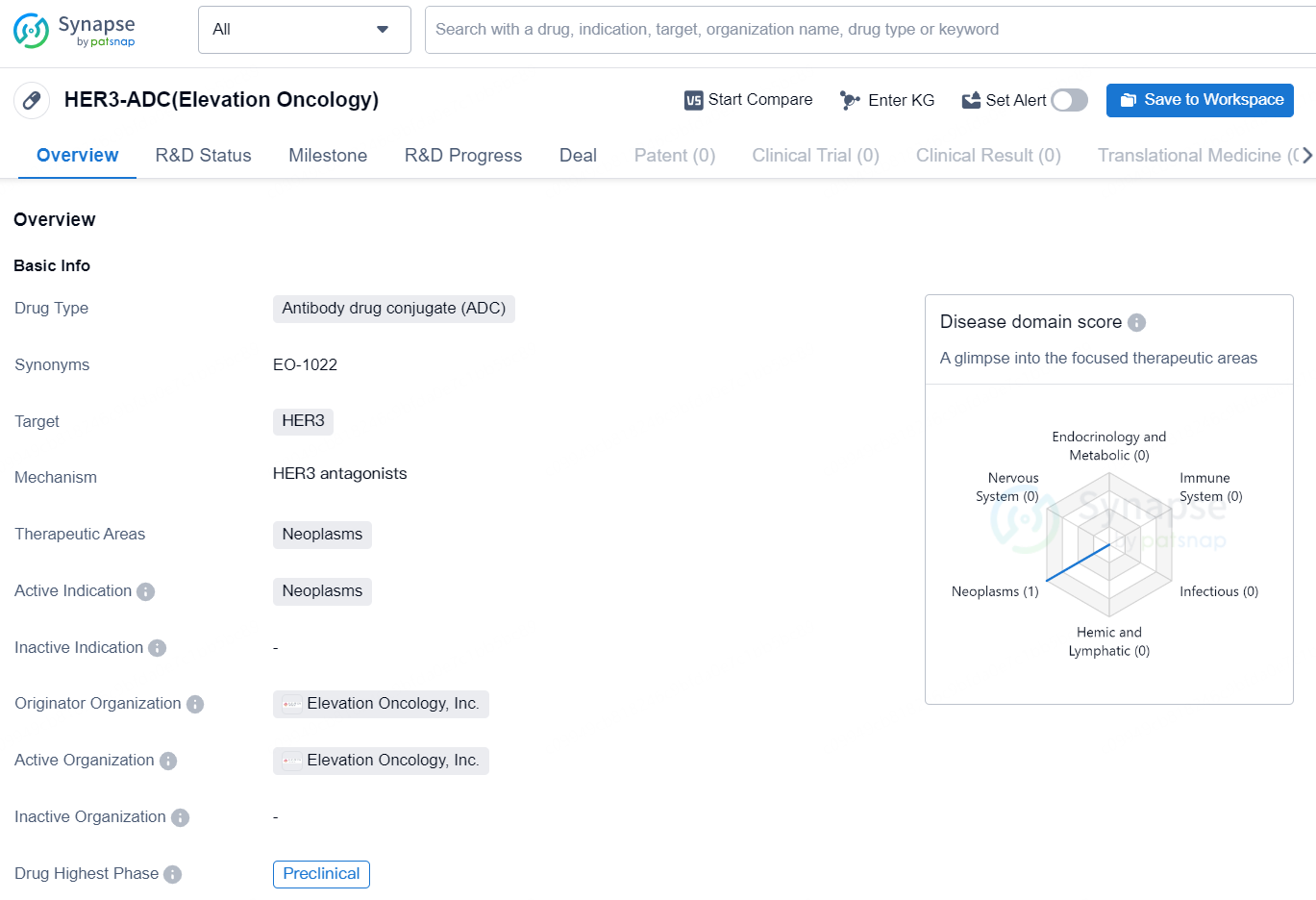
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
HER3 is a protein found in various solid tumors, such as breast cancer, non-small cell lung cancer with EGFR mutations, and pancreatic cancer, and is frequently linked to unfavorable clinical results. EO-1022 is an innovative antibody-drug conjugate (ADC) that incorporates seribantumab, an anti-HER3 monoclonal antibody (mAb), along with a payload of monomethyl auristatin E (MMAE), featuring site-directed conjugation to the glycan. This ADC is under development for patients with solid tumors that express HER3.
“The selection of our HER3-ADC candidate signifies a significant milestone for Elevation Oncology in enhancing our ADC portfolio. EO-1022 merges the seribantumab antibody with advanced site-specific ADC technology. We are confident that seribantumab is particularly suitable for use in an ADC, thanks to its specificity in targeting HER3-positive cancer cells with cytotoxic payloads and its favorable safety profile observed in over 900 patients across multiple trials,” stated Joseph Ferra, President and CEO of Elevation Oncology. “This initiative represents progress in utilizing our expertise in ADCs and oncology drug development to create innovative and targeted cancer treatments that meet considerable unmet medical needs. We anticipate presenting preclinical findings for EO-1022 in the first half of 2025 as we work towards bringing this asset into clinical trials.”
Elevation Oncology has announced that it has established a licensing agreement with Synaffix B.V. This agreement provides Elevation Oncology with global access to Synaffix’s clinical-stage, site-specific ADC technology platform, which includes the GlycoConnect® antibody conjugation technology, HydraSpace® polar spacer technology, and the toxSYN® linker-payload, SYNstatin E™. The license for GlycoConnect® and HydraSpace® technologies is exclusive to HER3 as a singular target in conjunction with the SYNstatin E® linker-payload.
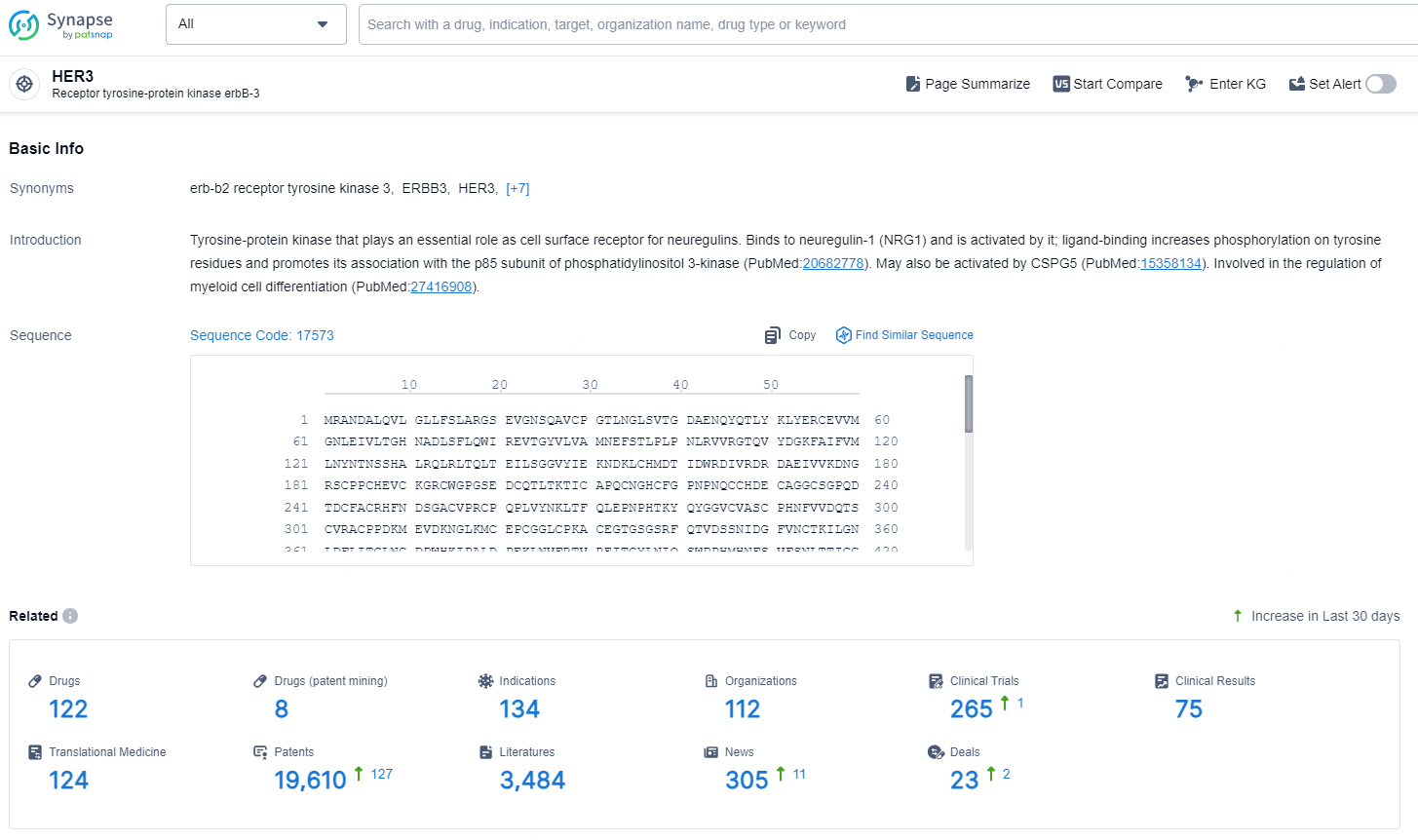
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Chemical, As of December 20, 2024, there are 122 investigational drugs for the HER3 target, including 134 indications, 112 R&D institutions involved, with related clinical trials reaching 265, and as many as 19610 patents.
The drug HER3-ADC (Elevation Oncology) is classified as an antibody drug conjugate (ADC) and is designed to target HER3, a protein associated with certain types of cancer. Specifically, HER3-ADC is intended for use in the treatment of neoplasms, which are abnormal growths of tissue that can be cancerous. It is currently in the preclinical phase of development, meaning that it has not yet been tested on humans and is still being evaluated in laboratory and animal studies.