Pfizer's IBRANCE® Boosts Progression-Free Survival in HR+/HER2+ Breast Cancer
Pfizer Inc. (NYSE:PFE) and Alliance Foundation Trials, LLC (AFT) have announced the findings from the Phase 3 PATINA trial, which indicates that incorporating IBRANCE® (palbociclib) into the existing standard first-line maintenance therapy following induction chemotherapy leads to a significant and clinically relevant enhancement in progression-free survival (PFS) based on investigator evaluation in patients with hormone receptor-positive (HR+), human epidermal growth factor receptor 2-positive (HER2+) metastatic breast cancer (MBC). Sponsored by AFT, the trial showed a median PFS of 44.3 months (95% CI: 32.4-60.9) for those receiving IBRANCE alongside anti-HER2 treatment (trastuzumab or trastuzumab plus pertuzumab) and endocrine therapy, in contrast to 29.1 months (95% CI: 23.3-38.6) for patients given only anti-HER2 therapy and endocrine therapy [HR: 0.74 (95% CI, 0.58-0.94); unstratified 1-sided p= 0.0074]. This indicates an increase in median PFS exceeding 15 months. The overall survival, which is a secondary endpoint, had not yet reached maturity during this analysis. These findings are being showcased in a late-breaking oral presentation (Abstract GS2-12) and featured in the press program at the 47th San Antonio Breast Cancer Symposium (SABCS) taking place in San Antonio, Texas.
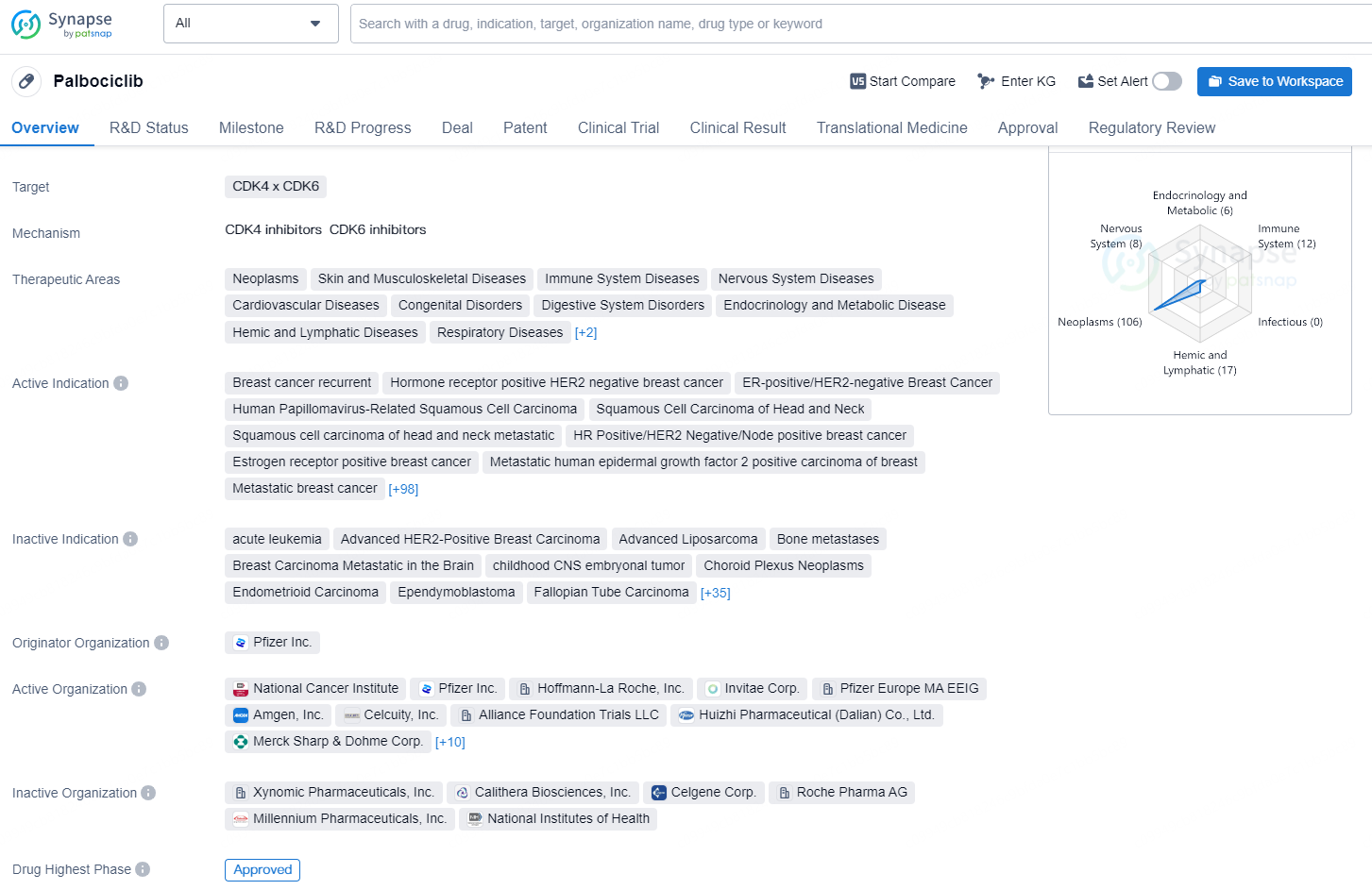
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"PATINA represents the inaugural extensive Phase 3 trial demonstrating the advantages of CDK4/6 inhibition in HR-positive, HER2-positive metastatic breast cancer," stated Otto Metzger, M.D., the lead investigator for Alliance Foundation Trials and a medical oncologist affiliated with the Dana-Farber Cancer Institute. "The findings support the possibility that this maintenance therapy can decelerate disease advancement and enhance clinical results in this group of patients."
Approximately 10% of breast cancer cases are categorized as HR+, HER2+, often referred to as double-positive or triple-positive breast cancers. Despite improvements in treatment options, the emergence of resistance to anti-HER2 and endocrine therapies poses a significant challenge, indicating the need for innovative therapeutic strategies for HR+, HER2+ metastatic breast cancer. Currently, IBRANCE is not approved for this specific condition.
"IBRANCE, the pioneering CDK4/6 inhibitor, has transformed the management of HR-positive, HER2-negative metastatic breast cancer, with over 773,000 patients treated since its initial approval in 2015," remarked Roger Dansey, M.D., Chief Development Officer for Oncology at Pfizer. "The data illustrates that integrating IBRANCE with standard care may serve as a promising maintenance therapy in HR-positive, HER2-positive malignancies. PATINA highlights Pfizer’s ongoing dedication to meeting the requirements of patients with breast cancer, and we anticipate discussing these findings with regulatory agencies."
In the PATINA trial, the safety and tolerability of IBRANCE were in line with its recognized safety profile for HR+, HER2-negative metastatic breast cancer, and no new safety concerns were noted. The predominant adverse effects associated with IBRANCE were hematologic issues, including neutropenia and leukopenia. Non-hematologic adverse events observed included fatigue, stomatitis, and diarrhea, generally ranging from mild to moderate in intensity.
Since its first regulatory authorization in 2015, IBRANCE has remained a standard first-line therapy for HR+, HER2-negative metastatic breast cancer and has received approval in over 108 nations. Pfizer intends to present the PATINA findings to regulatory authorities.
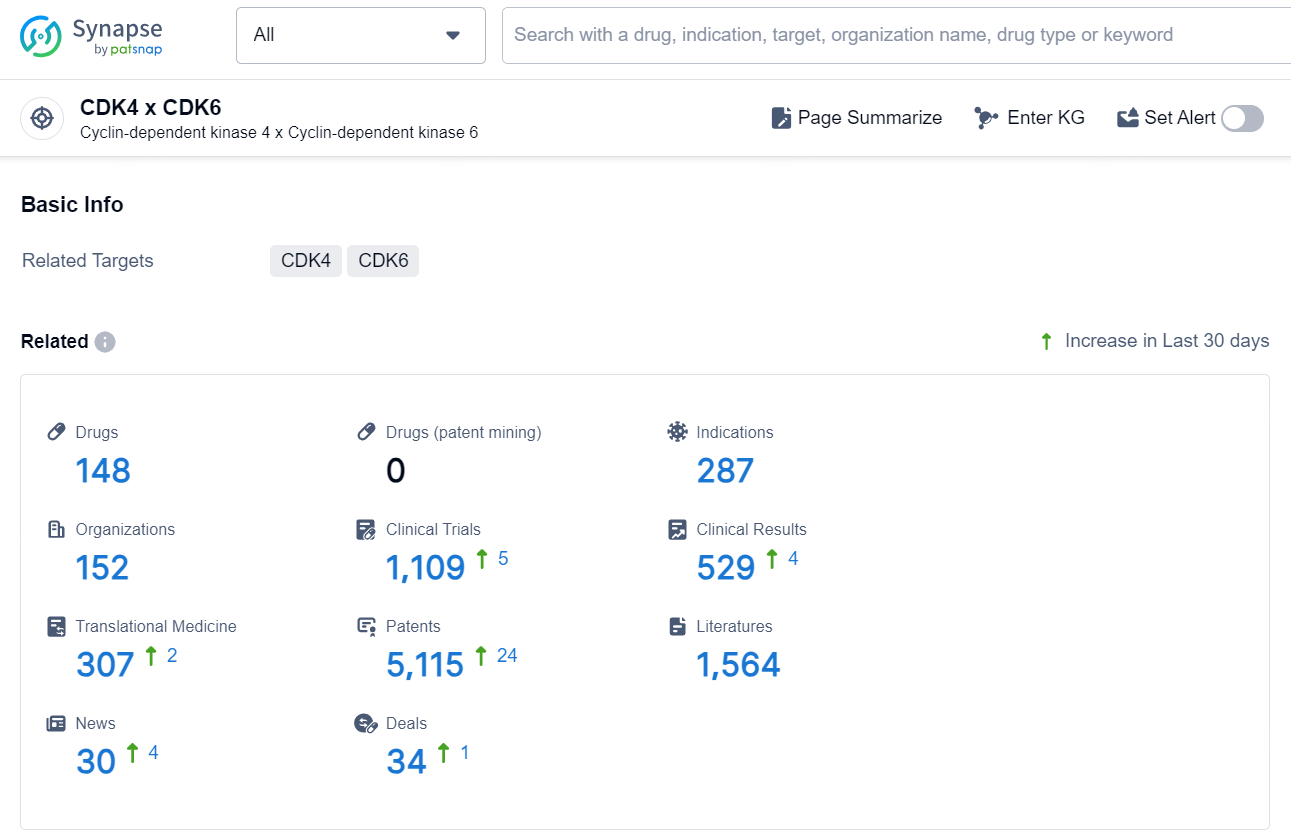
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of December 20, 2024, there are 148 investigational drugs for the CDK4 x CDK6 target, including 287 indications, 152 R&D institutions involved, with related clinical trials reaching 1109, and as many as 5115 patents.
Palbociclib is a small molecule drug that targets CDK4 and CDK6, and it has been approved for various therapeutic areas. The drug has been indicated for the treatment of numerous types of cancers, such as breast cancer, ovarian cancer, colorectal cancer, renal cell carcinoma, leukemia, non-small cell lung cancer, and lymphoma.