FDA Approves Neurocrine's CRENESSITY™ for Congenital Adrenal Hyperplasia
Neurocrine Biosciences, Inc. (Nasdaq: NBIX) has announced that the U.S. Food and Drug Administration has granted approval for CRENESSITY™ (crinecerfont) capsules and oral solution as an adjunct therapy to glucocorticoid replacement therapy aimed at managing androgen levels in both adult and pediatric patients aged four and above who have classic congenital adrenal hyperplasia (CAH), a serious and rare genetic disorder affecting the adrenal glands. CRENESSITY serves as a selective and powerful oral antagonist for the corticotropin-releasing factor type 1 receptor (CRF1), marking it as the first and only treatment for classic CAH that actively lowers excess adrenocorticotropic hormone (ACTH) alongside reducing the production of adrenal androgens. This advancement offers the potential for decreasing glucocorticoid dosages, representing a significant breakthrough in addressing classic CAH.
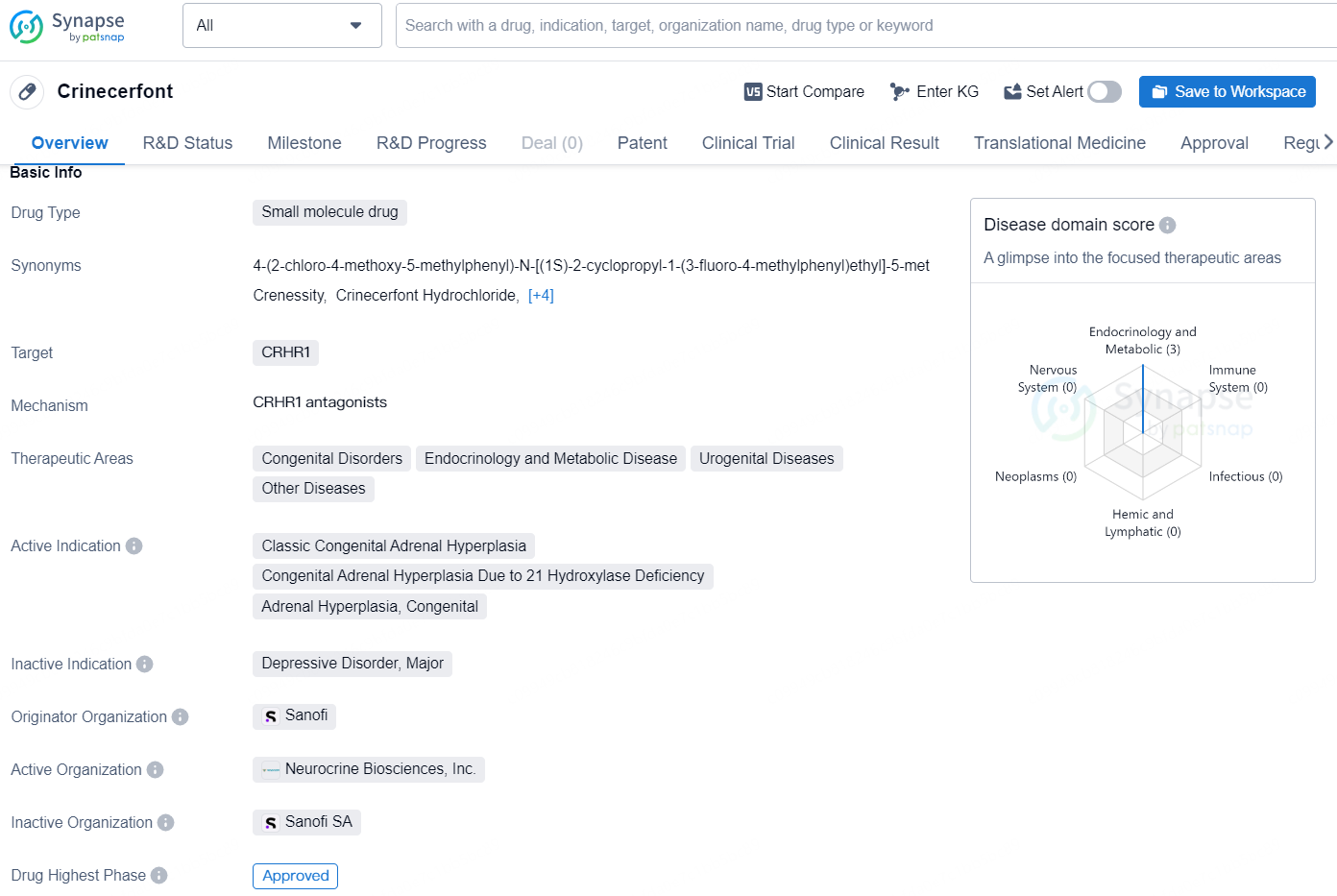
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
“For thirty years, Neurocrine Biosciences, alongside our late founder Wylie W. Vale, has led innovative research that has unveiled the vital function of corticotropin-releasing factor and its receptor, CRF1, in the pathophysiology of congenital adrenal hyperplasia (CAH),” stated Kyle W. Gano, Ph.D., Chief Executive Officer of Neurocrine Biosciences. “The approval of CRENESSITY marks an important achievement for the CAH community, and we extend our gratitude to the trial participants, their families, and the clinical researchers who contributed to the development of this new therapy and class of medications.”
“Patients and their families face challenges in balancing the management of CAH symptoms with the side effects or complications associated with high-dose steroid treatments, which can affect their quality of life,” remarked Dina Matos, Executive Director of the CARES Foundation. “We appreciate Neurocrine Biosciences' efforts to engage our community during the drug development process to grasp our needs and ultimately deliver a new treatment that can help decrease excess adrenal androgens and reduce reliance on high-dose steroids for those with CAH.”
CRENESSITY is anticipated to be on the market in about a week. The medication will be distributed through PANTHERx Rare, a specialized pharmacy, to streamline and simplify the prescription process for CRENESSITY.
Neurocrine Biosciences is dedicated to assisting patients in accessing CRENESSITY through Neurocrine Access Support, a complimentary and all-encompassing program designed for patients, caregivers, and healthcare providers. This program offers various resources to ensure that patients have the necessary support to start and maintain treatment with CRENESSITY. A dedicated Care Coordinator, supported by a team, is available to guide patients and caregivers through the insurance procedures and discover suitable financial aid opportunities. The majority of patients will pay $10 or less monthly for CRENESSITY*.
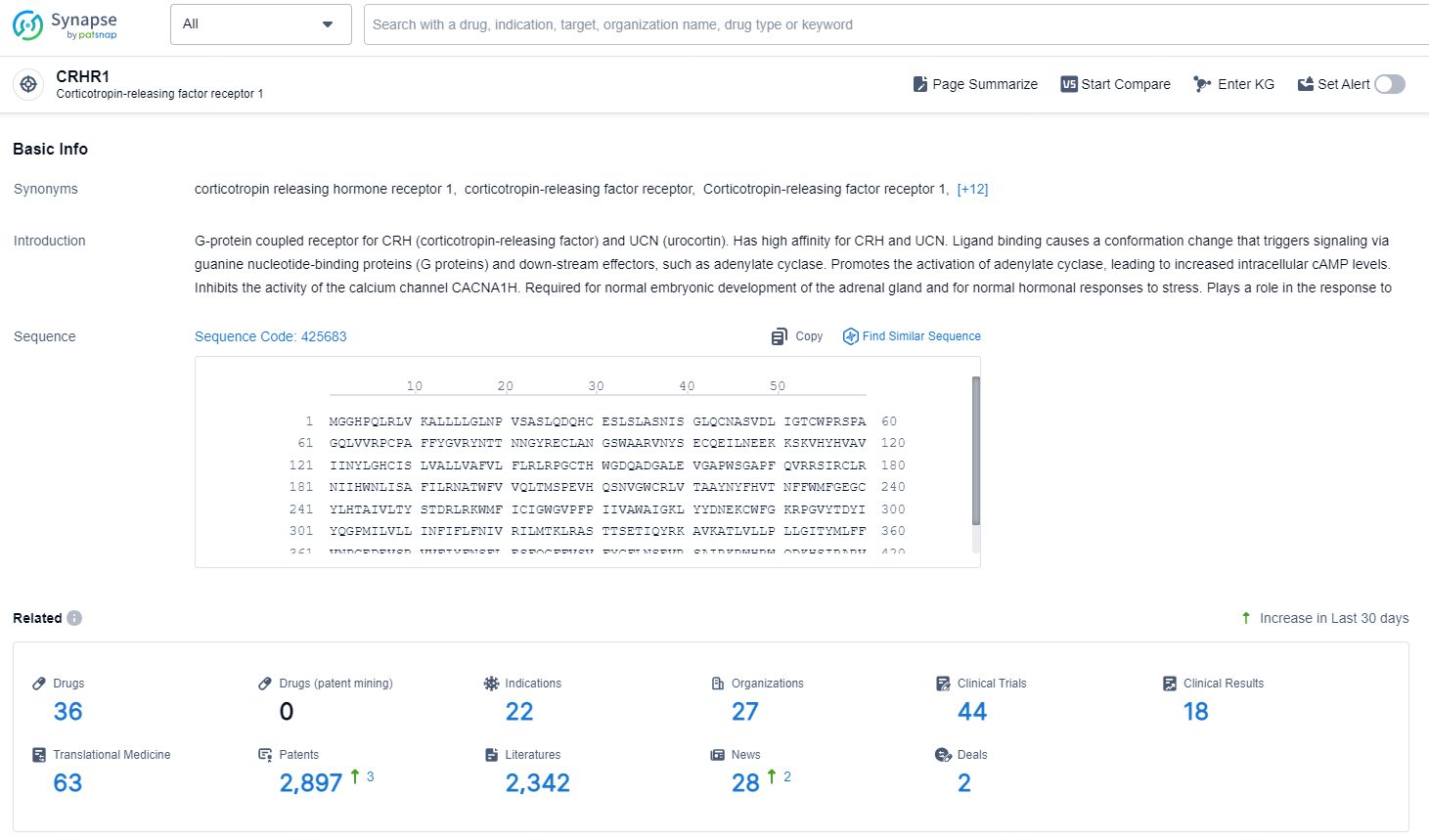
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Chemical, As of December 20, 2024, there are 36 investigational drugs for the CRHR1 target, including 22 indications, 27 R&D institutions involved, with related clinical trials reaching 44, and as many as 2897 patents.
Crinecerfont is a small molecule drug developed by Sanofi, designed to target CRHR1. The drug has received its highest phase of approval globally, with its first approval date set for December 2024, in the United States. Crinecerfont is indicated for the treatment of several therapeutic areas, including Congenital Disorders, Endocrinology and Metabolic Disease, Urogenital Diseases, and Other Diseases.