Corcept Reports Phase 2 Study Outcomes for Dazucorilant in ALS Patients
Corcept Therapeutics Incorporated (NASDAQ: CORT), a company at the commercial stage focusing on the discovery and development of drugs aimed at treating severe endocrinological, oncological, metabolic, and neurological disorders through the modulation of cortisol hormone effects, has announced findings from the DAZALS study. This study was a randomized, double-blind, placebo-controlled Phase 2 trial assessing two doses (150 mg and 300 mg) of its unique selective cortisol modulator, dazucorilant, in patients diagnosed with ALS. Following the trial's conclusion, participants were given the opportunity to join a long-term open-label extension study, where they received a dosage of 300 mg of dazucorilant.
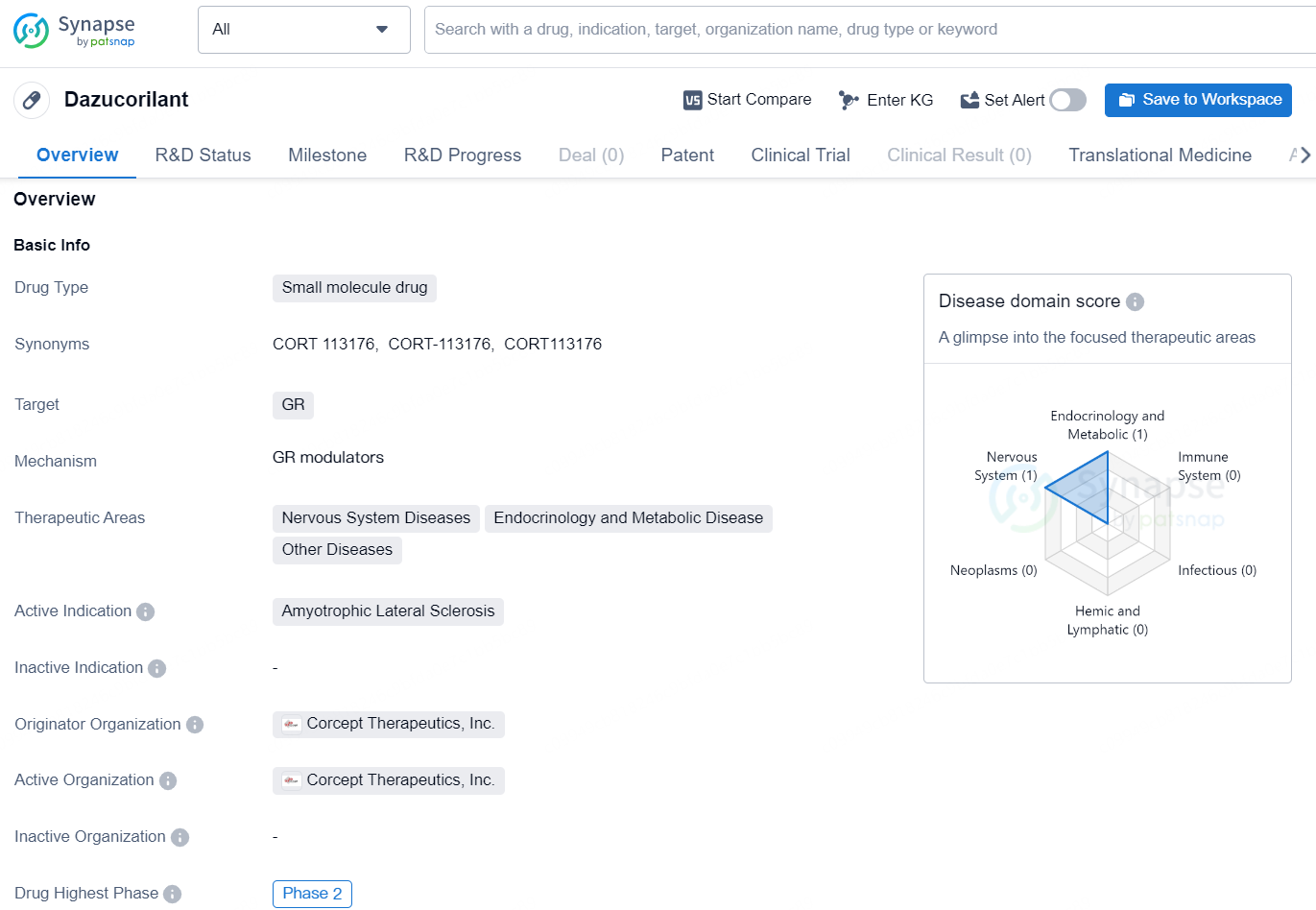
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
The DAZALS trial did not achieve its main goal, which was to measure the change from baseline in the ALS Functional Rating Scale-Revised (ALSFRS-R) among patients taking dazucorilant versus those on a placebo. Those treated with dazucorilant reported significantly higher levels of gastrointestinal discomfort at the beginning of the treatment compared to the placebo group. Over the course of the 24-week study, there were no fatalities (0 out of 83) in the 300 mg group, while the placebo group recorded 5 deaths (5 out of 82), yielding a p-value of 0.02. A long-term extension study, which is open-label, will proceed, with overall survival being evaluated in March 2025 after all participants have been treated for one year. The U.S. Food and Drug Administration has awarded Dazucorilant with Fast Track Designation. Full findings from the DAZALS trial are set to be shared at a medical conference in the following year.
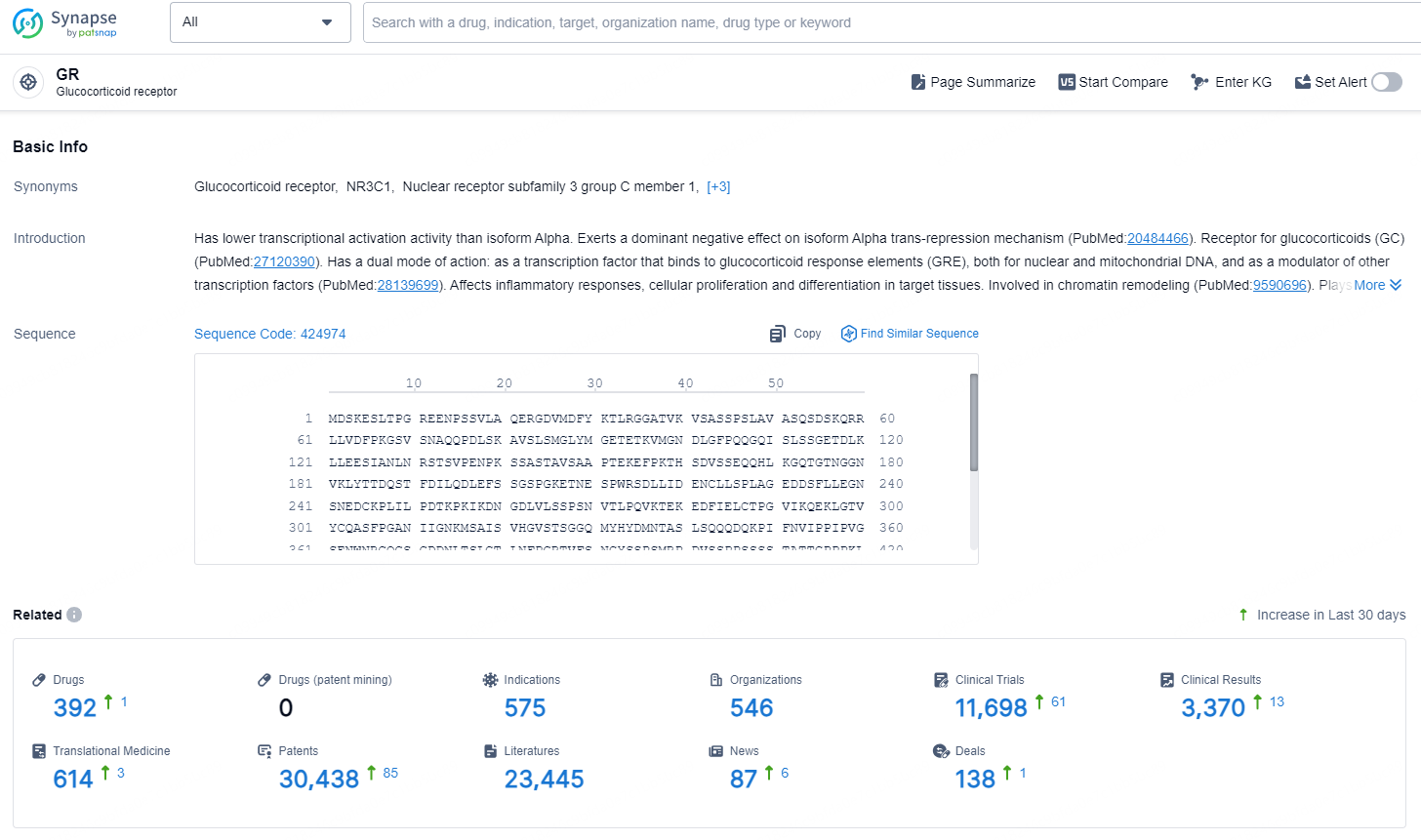
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of December 20, 2024, there are 392 investigational drugs for the GR target, including 575 indications, 546 R&D institutions involved, with related clinical trials reaching 11698, and as many as 30438 patents.
Dazucorilant is a small molecule drug developed by Corcept Therapeutics, Inc. The drug targets the GR (glucocorticoid receptor) and is currently in Phase 2 of clinical development. It is primarily being investigated for the treatment of Amyotrophic Lateral Sclerosis (ALS), also known as Lou Gehrig's disease.