siRNA Drug Amvuttra Patent Research and Practical Operation Guide(3)
Based on the characteristics of siRNA drugs, we have summarized the process for conducting patent research on siRNA drugs. You can start by reading the following article to get background information.
siRNA Drug Amvuttra Patent Research and Practical Operation Guide(1)
siRNA Drug Amvuttra Patent Research and Practical Operation Guide(2)
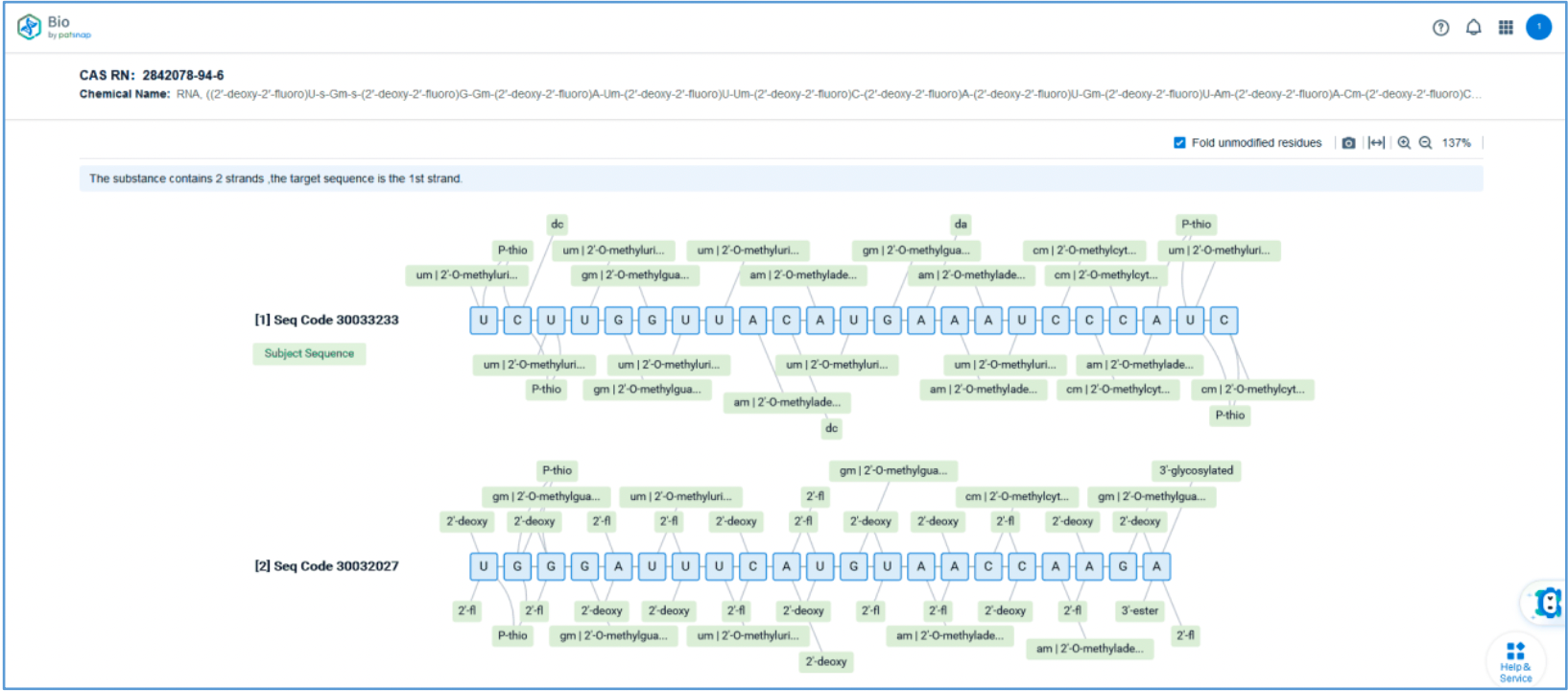
Retrieval of Sense/Antisense Strand Sequences
First, log in to Patsnap Bio and select the "Sequences" search option. Then, run two searches using the sense strand sequence and antisense strand sequence of Amvuttra. Next, filter the patents as needed and save them to the Workspace created in step 2.3. The sequence information in this search can also be compared with that obtained in step 2.2 to further determine the accuracy of the retrieval.
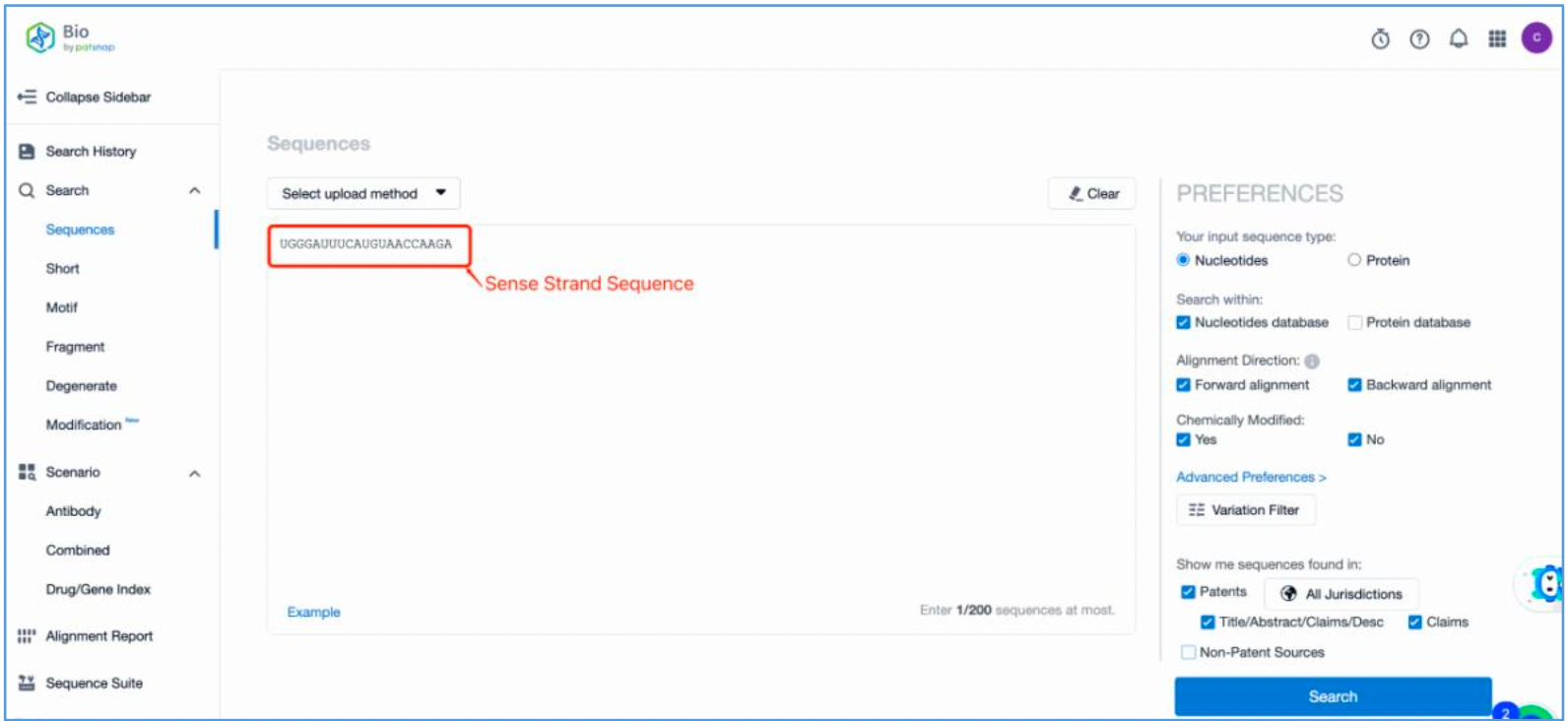
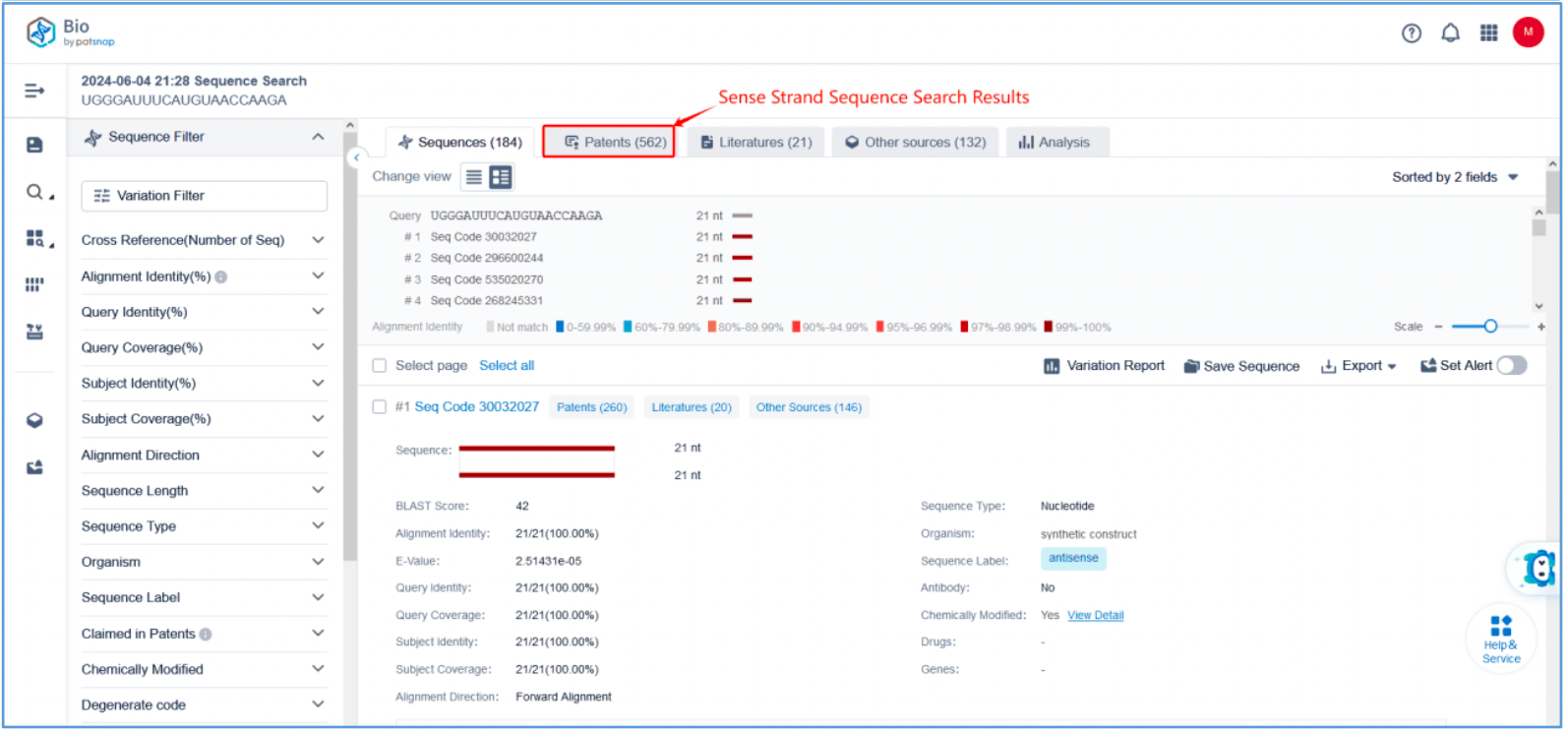
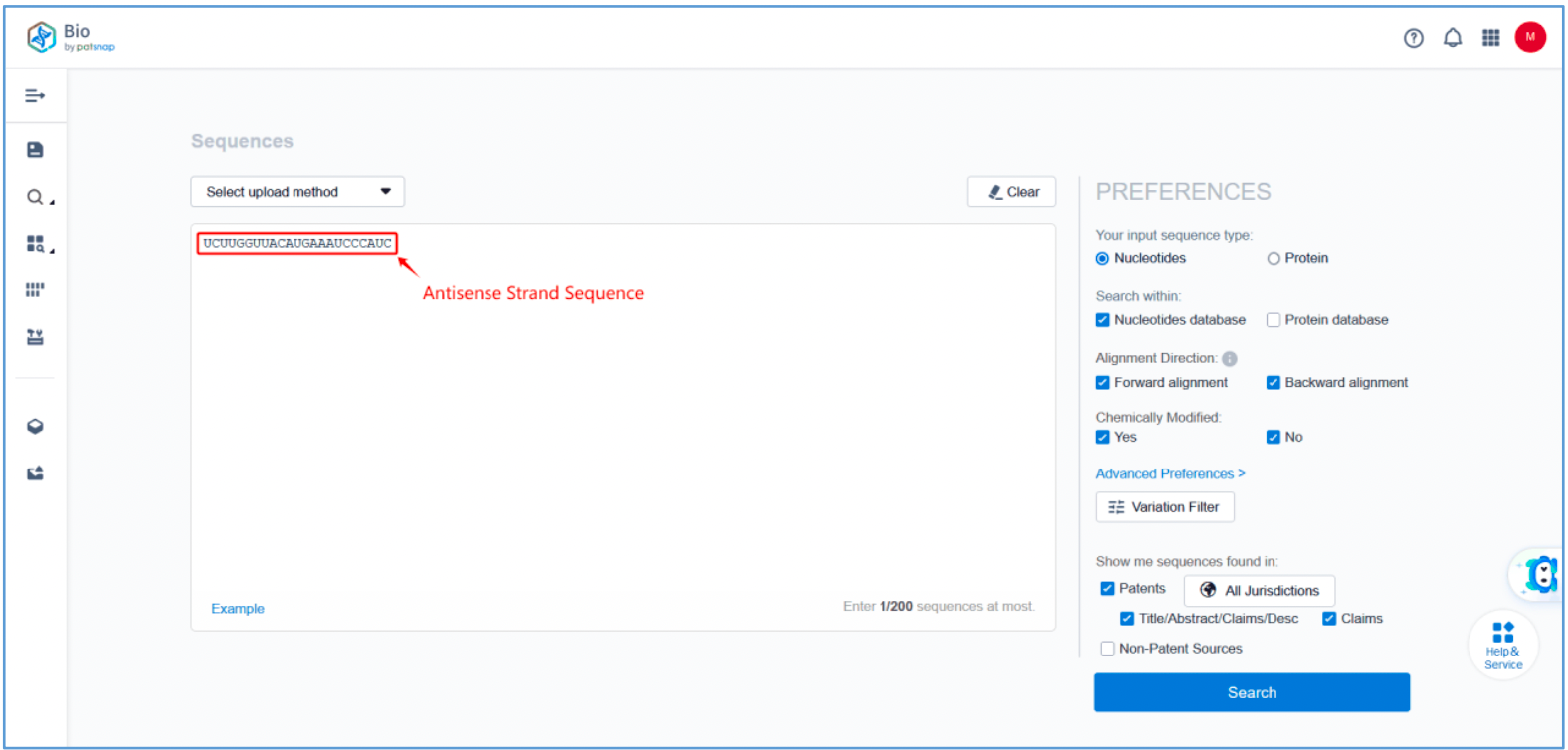
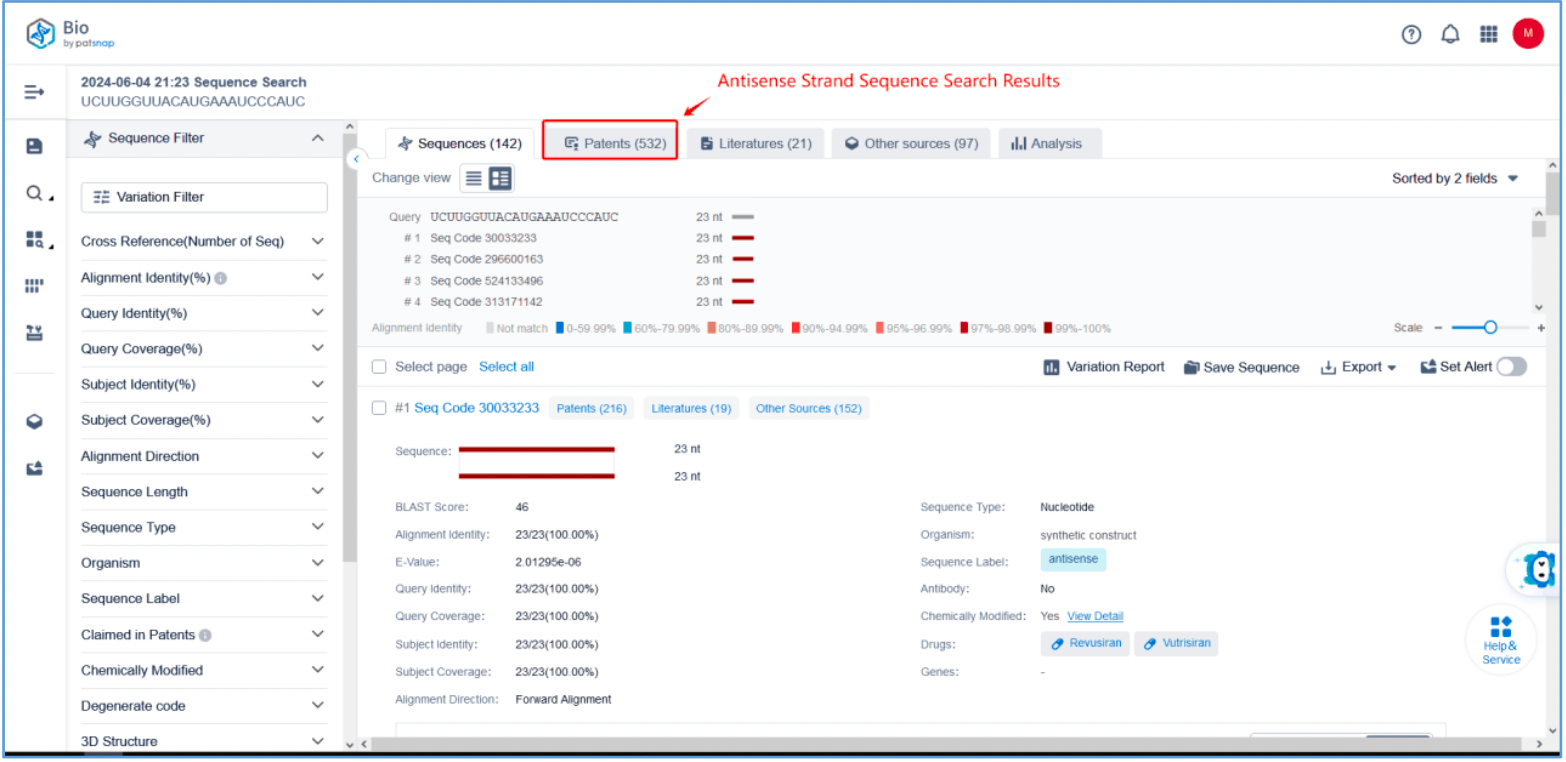
Retrieval of Sense/Antisense Strand Modifications
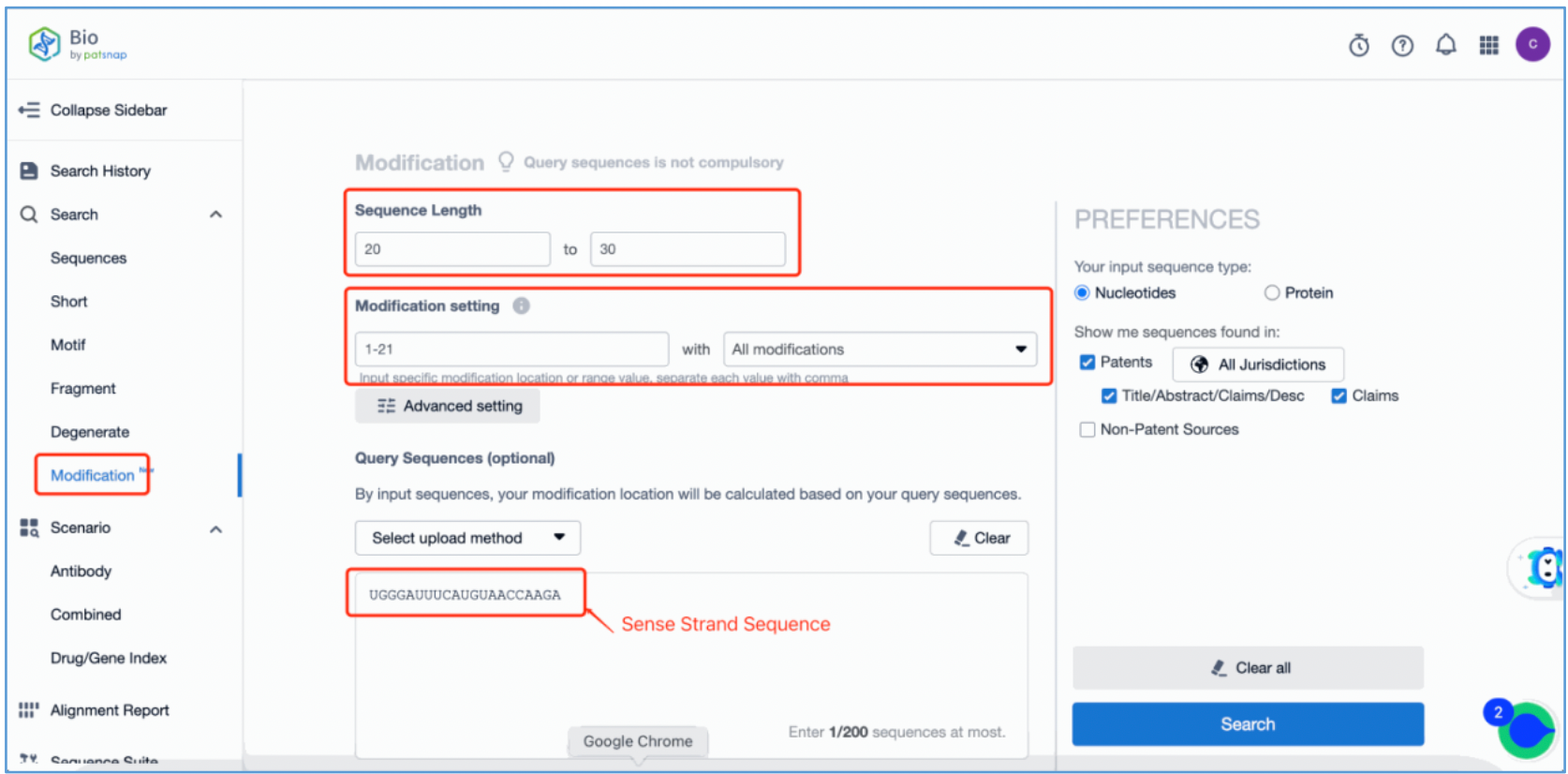
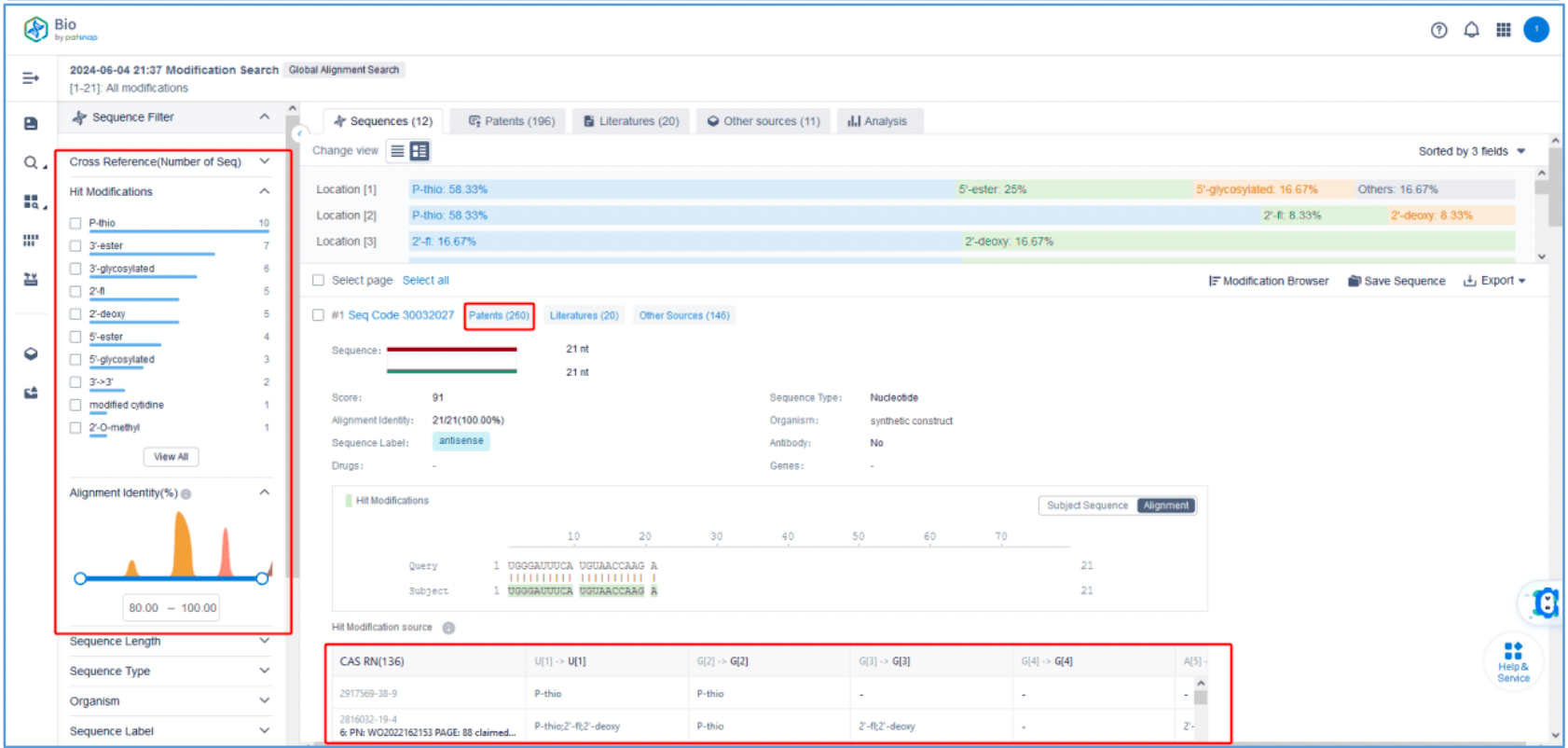
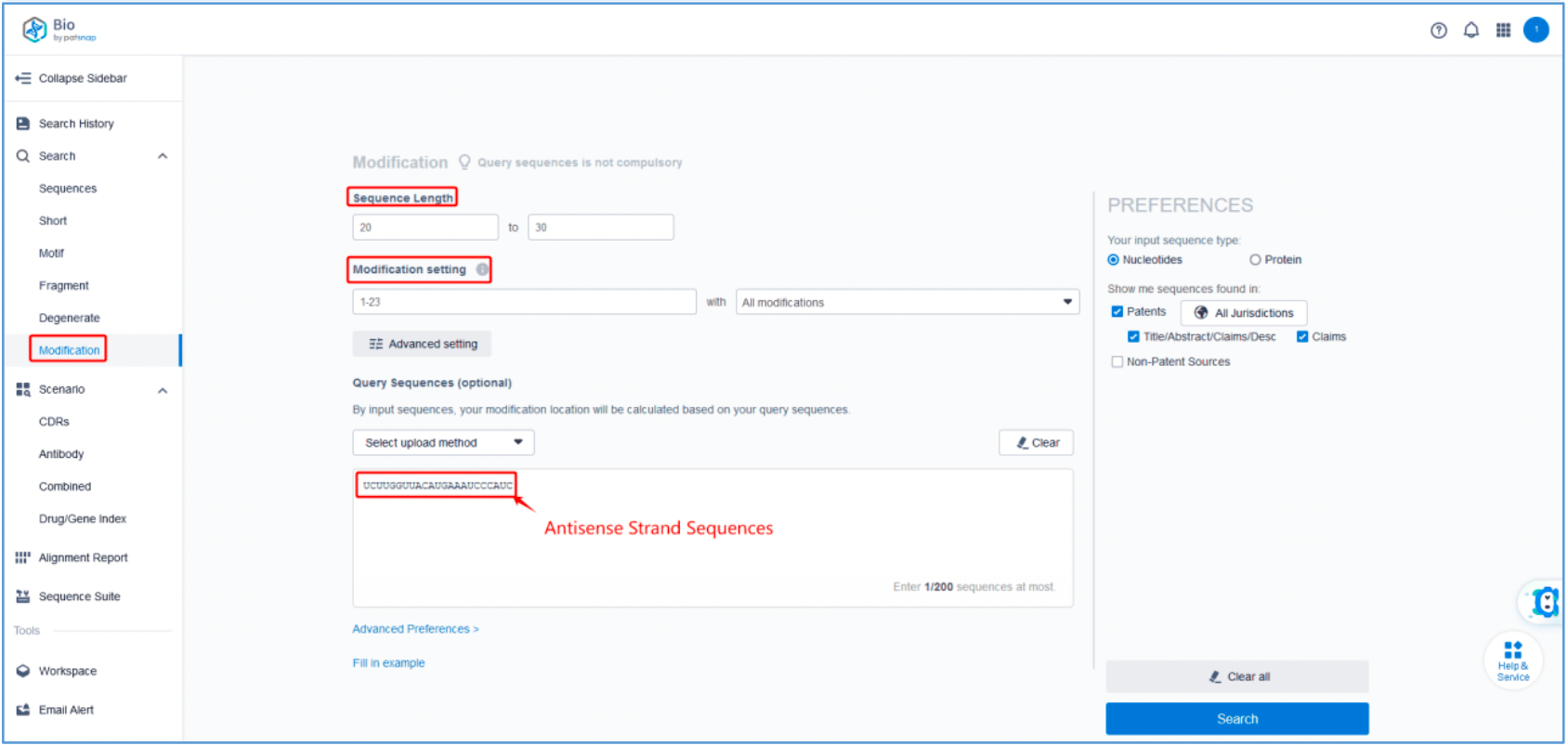
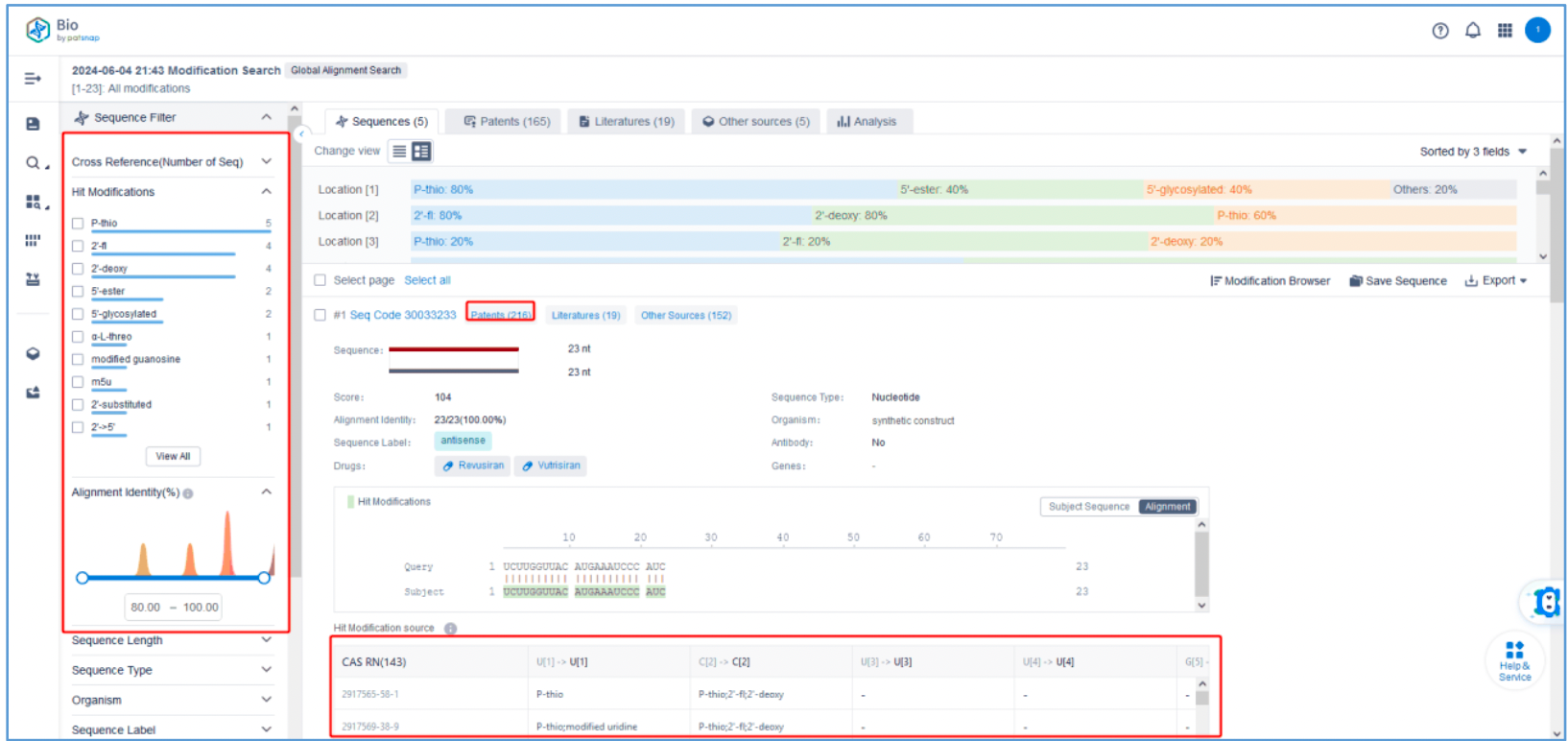
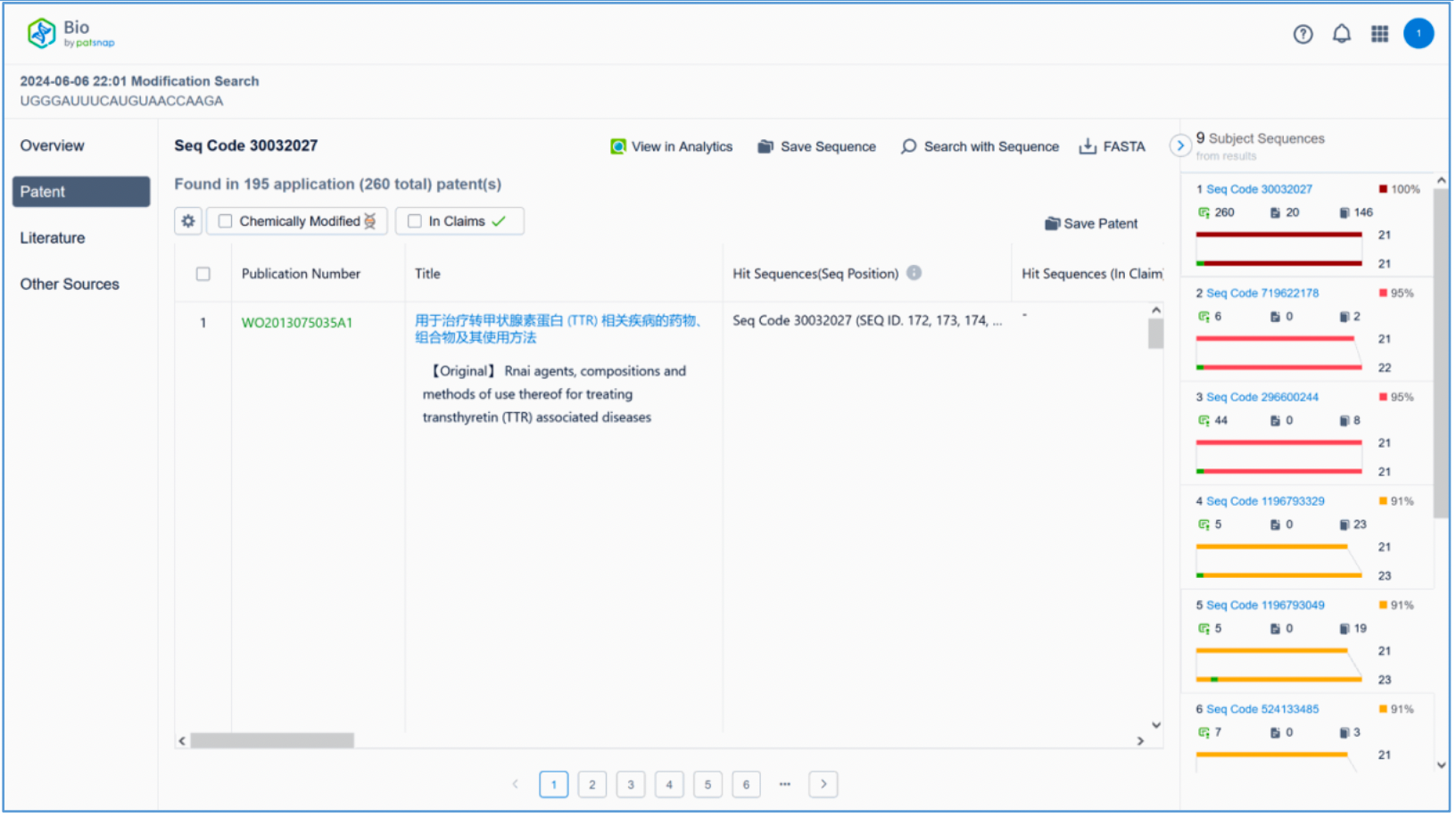
To retrieve modification data, select the "Modification" search option. Run two searches using the sense strand sequence and antisense strand sequence of Amvuttra, with the required inputs for "Sequence Length" and "Modification Setting".
Refine the results using the sequence filters, hover over highlighted nucleotides to display their specific modifications, view all patents involved in the sequence search results, and check the modification status of a specific sequence by the CAS registration number. Finally, save the refined results to Workspace.
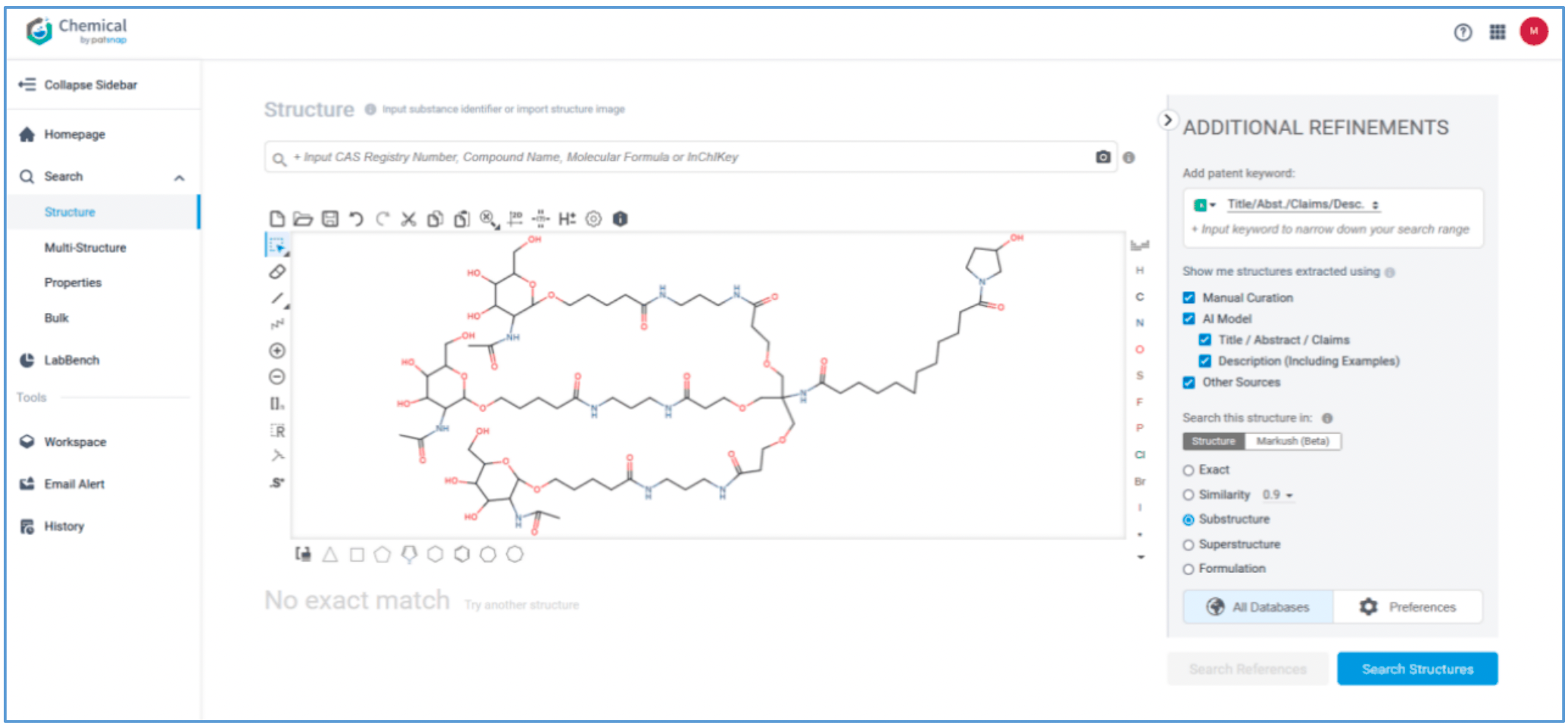
Retrieval of Delivery Agents
Amvuttra’s delivery vehicle is a ligand composed of three N-acetygalactosamine (GalNAc) residues. The conventional method of retrieving the ligand and its patents would be through a keyword search combining keywords‘siRNA’and‘acetylgalactosamine’with the related classification number. This guide demonstrates how to retrieve the ligand and its patents using a structure search.
First, log in to Patsnap Chemical and select Structure search. Draw or import Amvuttra’s ligand structure in the structure editor. Select Substructure Search in the additional refinements, and click Search Structures. Next, select the corresponding structure in the search results and click “View in Analytics” to view the 117 related patents. Filter the patents as needed and save them to Workspace.
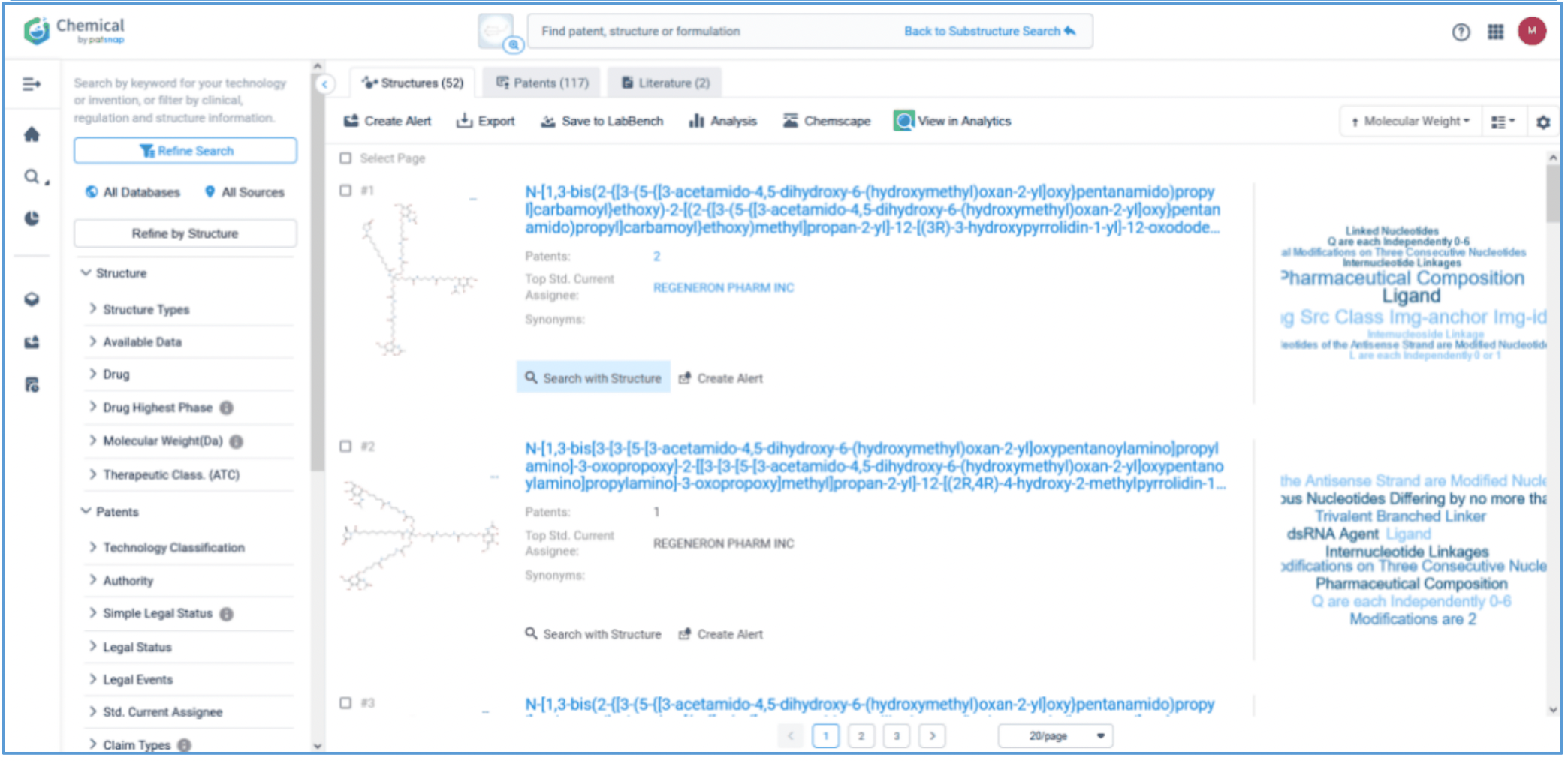
Analyzing and Mapping All Patents Related to Amvuttra
Next, analyze all patents saved to the "Amvuttra" workspace in steps 2.3 and 2.5 and perform a simple family expansion to avoid missing key patents. To analyze Amvuttra's patent layout in the United States and China based on the application date, filter the patents for each respective company in Workspace, and create separate folders.
Next, sort the patents in each subfolder in ascending order of its application date, and review each patent’s claims and specifications to classify their main protection themes, patent legal status, and application date. The following patent layout diagrams in the United States and China based on application date are generated:
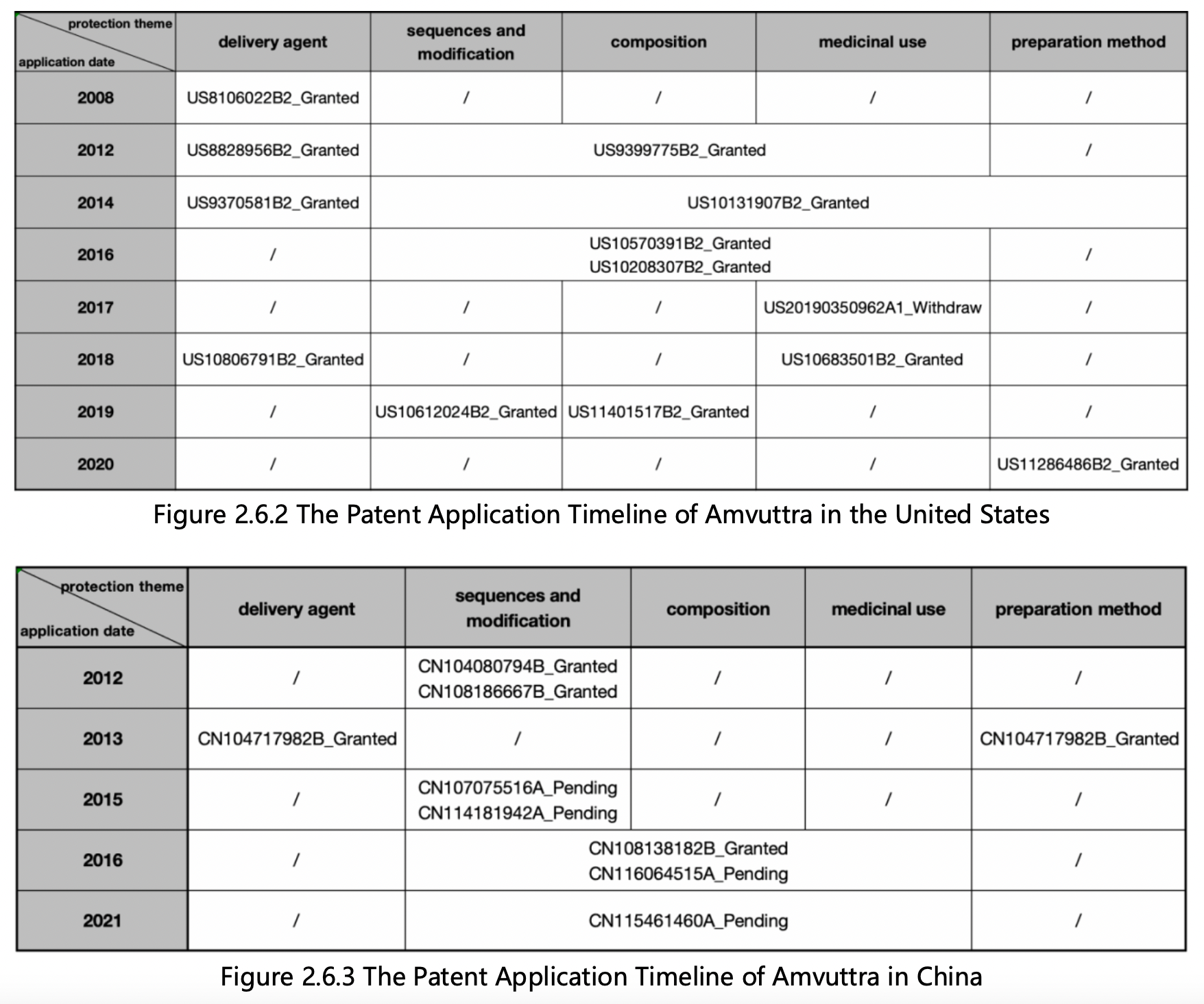
Based on Figures 2.6.2 and 2.6.3, the features of the patent protection and components of Amvuttra in the United States and China are as follows:
1. Alnylam's US basic patent for protecting Amvuttra delivery agents is US8106022B2, which was applied for in 2008 and is still in the protection period. Subsequently, there are also independent claims for protecting delivery agents in US patents US8828956B2, US9370581B2, and US10806791B2. The earliest Chinese patent application for protecting Amvuttra delivery agents was CN102006890A, which was applied for in 2008 but not granted. Subsequently, CN104080794B, which was applied for in 2013 and is still in the protection period, protected Amvuttra delivery agents and their preparation method. In addition, protecting delivery agents are only involved in the patents of the main protection sequences such as CN108138182B, CN116064515A, CN115461460A, as well as dependent claims of the patent application. Therefore, Alnylam has formed a continuous and comprehensive patent protection strategy for Amvuttra delivery agents in the United States, while its patent layout in China is slightly weaker.
2.Alnylam's US basic patent US9399775B2 for protecting Amvuttra sequences and its modifications was applied for in 2012 and is still in the protection period. Subsequently, it applied for multiple patents in the United States to ensure comprehensive protection of its composition, medicinal use, and preparation method, including US10131907B2, US10570391B2, US10208307B2, US20190350962A1, US10683501B2, US10612024B2, US11401517B2, US11286486B2, etc. With the exception of US20190350962A1, which was withdrawn, all the US patents are still in the protection period. In China, Alnylam's patent layout for Amvuttra's sequences and modifications, composition, and medicinal use is slightly lagging compared to that of the US. It has carried out a more comprehensive layout since 2015. With the exception of CN108138182B, which was granted, all the Chinese patents are currently in the substantive examination stage. Referring to the examination of patent families in the US, it is expected that patent applications in China have a relatively high prospect of being granted.
3.Since applying for patents to protect the core components of Amvuttra delivery agents, sequences, and their modifications, Alnylam has filed many divisional and continuation applications. Currently, it has obtained 31 US-licensed patents and 4 Chinese-licensed patents, which not only expands the scope and intensity of protection, but also further extends the protection period of patents through priority. Alnylam's patent protection strategy for Amvuttra is a typical example for siRNA drugs, and this guide demonstrated the core methodology of retrieving similar drugs and how to assess their patent protection strategy.
Summary
This guide outlines the process of siRNA drug patent research and analysis of Alnylam's Amvuttra patent protection strategy. Throughout the retrieval process, various online intelligence platforms such as Patsnap Analytics, Synapse, Bio and Chemical were utilized. This guide demonstrates that patent research is a multi-stage, collaborative endeavor that involves determining retrieval elements, conducting patent retrieval, analyzing core patents, and conducting supplementary retrieval based on drug profiles. By obtaining the patent layout of Amvuttra in the United States and China, its protection history was quick to assess, and its future progress could be extrapolated. This step-by-step guide can be applied to similar siRNA drug patent research to better enable companies in infringement analysis, biosimilar development, investment, and financing.
For more information, please click the image link below to access the full report.




