Experimental Seladelpar Shows Promise in Treating Liver Disease and Itching in PBC
Gilead Sciences, Inc. recently acquired CymaBay Therapeutics, Inc. and has since unveiled interim data from the ongoing ASSURE trial. The results indicate that treatment with seladelpar, an investigational PPAR delta agonist, leads to improved cholestasis markers and decreased inflammation. Moreover, additional findings show that seladelpar can aid in reducing pruritus for individuals with primary biliary cholangitis.
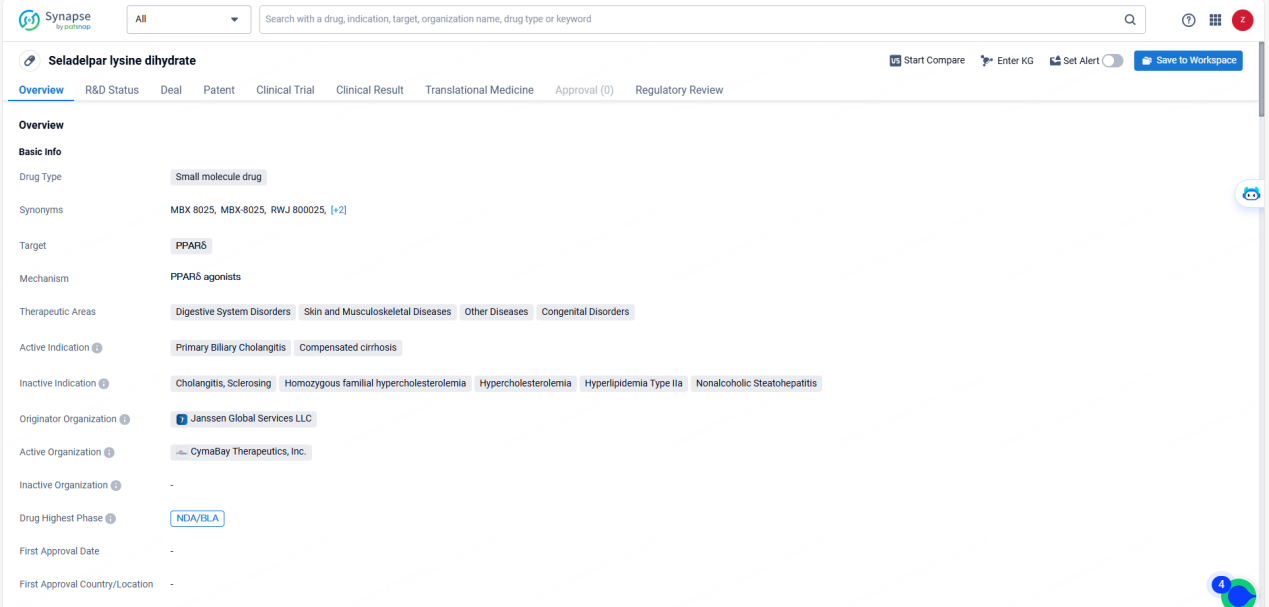
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Currently, there are no approved treatments for PBC-related pruritis. Findings from this study will be presented orally during the Presidential Plenary Session at the Digestive Disease Week 2024 Conference in Washington, DC.
ASSURE is an open-label trial assessing the long-term safety and effectiveness of seladelpar, a potent and selective peroxisome proliferator-activated receptor delta agonist, administered once daily. The trial involved adult PBC patients who had previously participated in seladelpar studies, with eligibility largely based on insufficient response to or intolerance of ursodeoxycholic acid.
This interim analysis excluded participants from the Phase 3 RESPONSE trial, which will be disclosed separately. Among the 174 participants included, most experienced a hiatus of at least one year between the end of their initial study and the commencement of ASSURE. Participants were given an open-label oral dose of 10 mg seladelpar daily, with most also continuing UDCA therapy.
“We are heartened by the positive interim data from ASSURE, aligning well with the findings from the Phase 3 randomized, double-blind, placebo-controlled RESPONSE study of seladelpar in patients who show inadequate response or intolerance to UDCA,” noted Cynthia Levy, M.D., Professor of Medicine at the University of Miami and study presenter.
“Seladelpar persistently shows significant effects on PBC liver disease, aids a substantial number of patients in normalizing their ALP levels, and notably reduces pruritus, a frequent and distressing comorbidity. Its safety and tolerability profile remains consistent with earlier PBC studies, emphasizing its potential as a promising treatment for PBC patients." Cynthia Levy added.
“The initial ASSURE data further corroborate the efficacy and safety profile of seladelpar observed throughout the comprehensive development programs, indicating that seladelpar could emerge as a leading therapy to significantly alter the treatment landscape for individuals with primary biliary cholangitis,” stated Merdad Parsey, Chief Medical Officer at Gilead Sciences.
The U.S. Food and Drug Administration has accepted the New Drug Application for seladelpar aimed at treating PBC, including pruritus, in adults without cirrhosis or with compensated cirrhosis who do not adequately respond to or cannot tolerate UDCA, with an expected decision in August 2024. The UK's Medicines and Healthcare products Regulatory Agency and the European Medicines Agency have also accepted seladelpar for review.
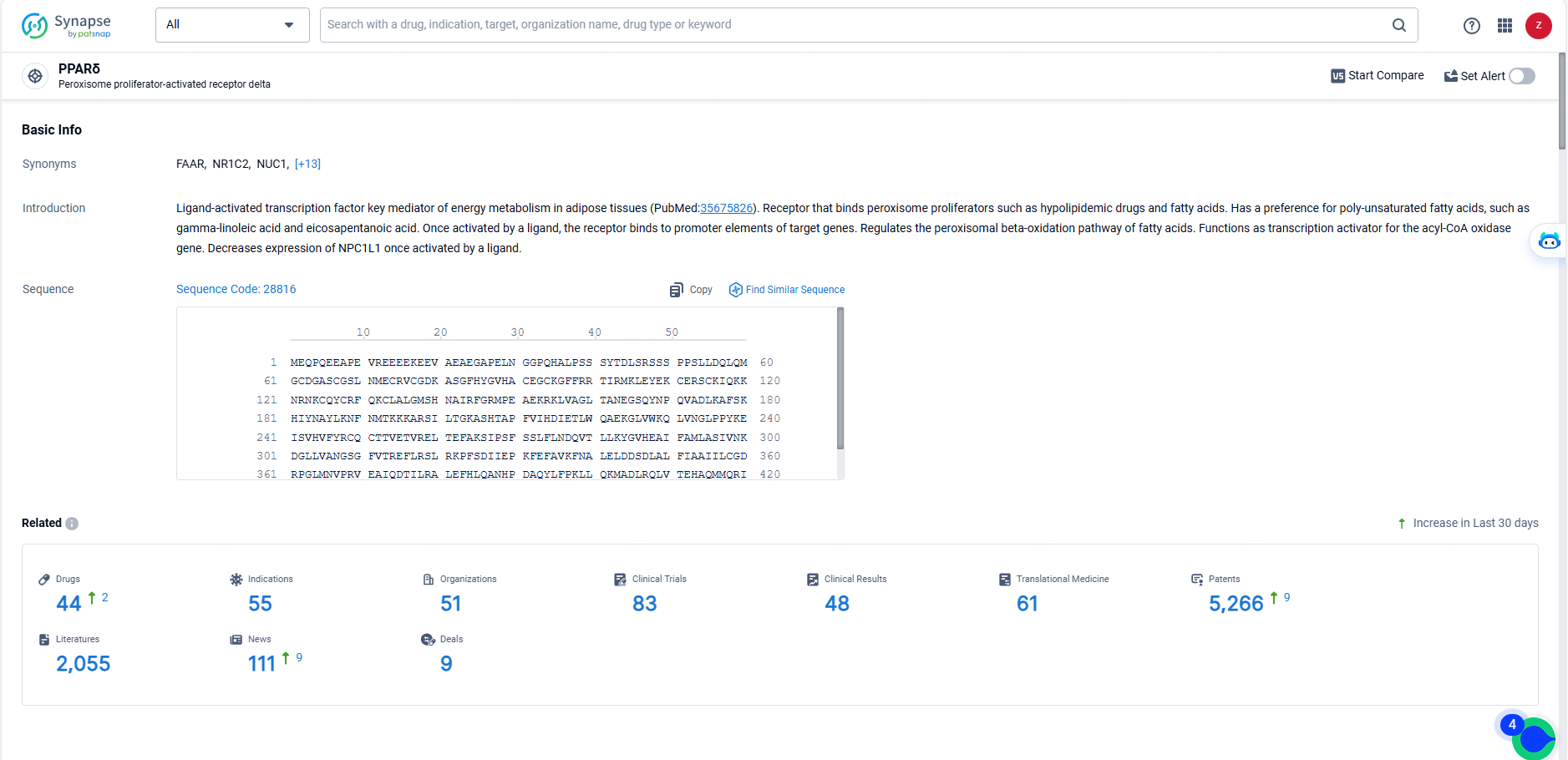
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of May 23, 2024, there are 44 investigational drugs for the PPAR delta target, including 55 indications, 51 R&D institutions involved, with related clinical trials reaching 83, and as many as 5266 patents.
Seladelpar lysine dihydrate shows promise as a potential treatment for primary biliary cholangitis and compensated cirrhosis, as well as other related therapeutic areas. The regulatory designations it has received further support its potential to address unmet medical needs and provide significant benefits to patients. As the drug progresses through the approval process, it will be important to continue monitoring its development and potential impact on the pharmaceutical industry and patient care.