Exploring Etravirine's Revolutionary R&D Successes and its Mechanism of Action on Drug Target
Etravirine's R&D Progress
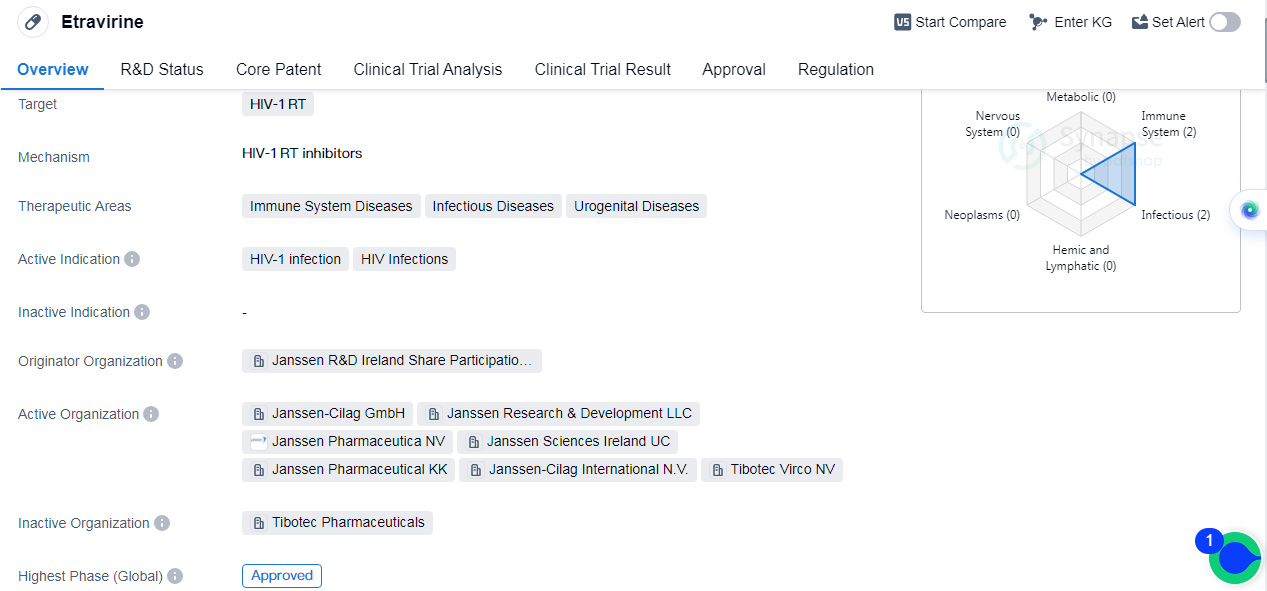
Etravirine is a small molecule drug that is primarily used for the treatment of HIV-1 infection. It specifically targets the HIV-1 reverse transcriptase (RT), which is an enzyme essential for the replication of the virus. By inhibiting the activity of this enzyme, Etravirine helps to suppress the replication of the virus and reduce the viral load in patients.
Etravirine has been approved for use in immune system diseases, infectious diseases, and urogenital diseases. The drug received its first approval in the United States in January 2008, making it available for use in the country. It has also obtained approval in other countries, suggesting its recognition and acceptance as a viable treatment option for HIV-1 infection.
The drug was developed by Janssen R&D Ireland Share Participation Scheme, an organization known for its contributions to the pharmaceutical industry.
Etravirine has reached the highest phase of development, with global approval. This signifies that it has successfully undergone rigorous testing and evaluation to demonstrate its safety and efficacy.
Furthermore, Etravirine is regulated as an orphan drug. This designation provides certain incentives and benefits to the manufacturer, such as market exclusivity and financial support, to encourage the development of treatments for rare diseases.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Etravirine: HSP90B inhibitors
HSP90B inhibitors are a type of drugs that target and inhibit the activity of heat shock protein 90 beta (HSP90B). HSP90B is a molecular chaperone protein that plays a crucial role in maintaining the stability and function of various client proteins involved in cell signaling, growth, and survival. By inhibiting HSP90B, these inhibitors disrupt the chaperone function of HSP90B and lead to the degradation of client proteins, ultimately affecting cellular processes.
From a biomedical perspective, HSP90B inhibitors have gained significant attention in cancer research. Many client proteins of HSP90B are involved in promoting the growth and survival of cancer cells. By inhibiting HSP90B, these inhibitors can potentially block the activity of these client proteins, leading to the suppression of cancer cell growth and proliferation. Therefore, HSP90B inhibitors are being investigated as potential anticancer agents and are being studied in preclinical and clinical trials.
It is important to note that HSP90B inhibitors may have off-target effects and can affect the function of other proteins in addition to HSP90B. Therefore, extensive research is being conducted to understand the specificity and selectivity of these inhibitors to minimize potential side effects and optimize their therapeutic potential.
Drug Target R&D Trends for Etravirine
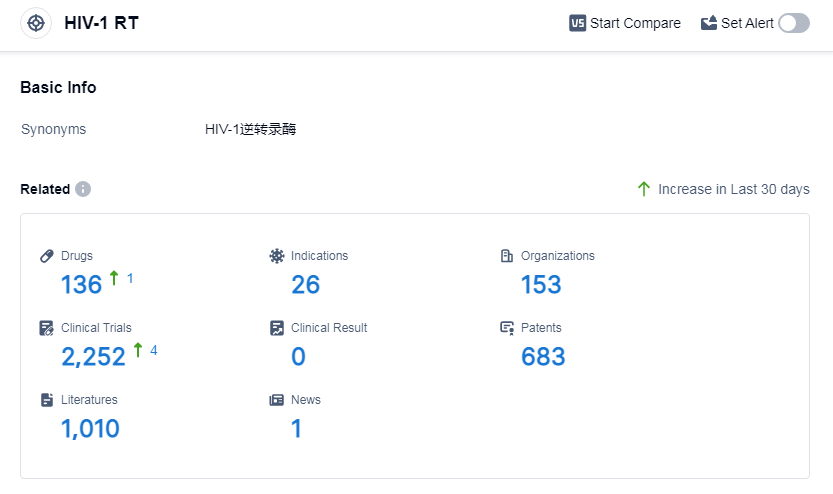
According to Patsnap Synapse, as of 6 Sep 2023, there are a total of 136 HIV-1 RT drugs worldwide, from 153 organizations, covering 26 indications, and conducting 2252 clinical trials.
Based on the analysis of the data provided, Gilead Sciences, Inc. is the company with the highest number of drugs under the target HIV-1 RT. The indications of HIV Infections and HIV-1 infection have the highest number of approved drugs. Small molecule drugs are the most common drug type under development. The European Union, United States, China, and Japan are the countries/locations with the highest number of drugs in development. Overall, the target HIV-1 RT has a competitive landscape with multiple companies and countries actively involved in research and development. The future development of this target is promising, with ongoing efforts to address various indications related to HIV and other diseases.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Etravirine is a small molecule drug that targets HIV-1 RT and is approved for the treatment of HIV-1 infection. It has demonstrated efficacy in multiple therapeutic areas and has obtained global approval. Developed by Janssen R&D Ireland Share Participation Scheme, Etravirine is regulated as an orphan drug, highlighting its potential to address unmet medical needs in the field of biomedicine.