Exploring Oxiconazole Nitrate's Revolutionary R&D Successes and its Mechanism of Action on Drug Target
Oxiconazole Nitrate's R&D Progress
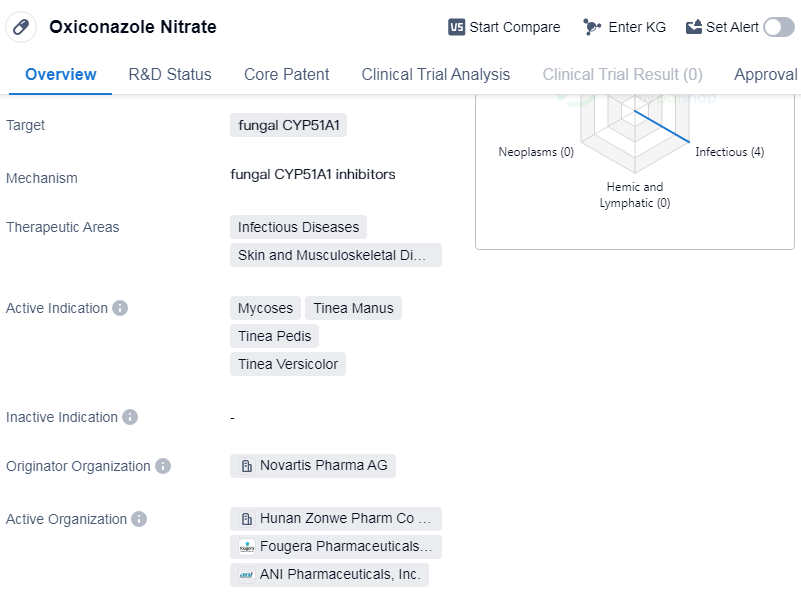
Oxiconazole Nitrate is a small molecule drug that falls under the therapeutic areas of infectious diseases, skin, and musculoskeletal diseases. It specifically targets fungal CYP51A1, making it effective against various fungal infections. The drug is primarily indicated for the treatment of mycoses, tinea manus, tinea pedis, and tinea versicolor.
The originator organization of Oxiconazole Nitrate is Novartis Pharma AG, a renowned pharmaceutical company. The drug has achieved the highest phase of approval in the global markets, indicating its safety and efficacy. It received its first approval in the United States in December 1988, making it a well-established drug in the market.
Oxiconazole Nitrate's mechanism of action involves inhibiting fungal CYP51A1, an enzyme essential for the synthesis of ergosterol, a crucial component of fungal cell membranes. By disrupting ergosterol production, the drug effectively weakens and kills the fungal cells, leading to the resolution of the infection.
The therapeutic areas targeted by Oxiconazole Nitrate highlight its broad spectrum of activity against infectious diseases, particularly those caused by fungi.
The approval of Oxiconazole Nitrate in multiple countries, including the United States and China, demonstrates its global recognition and acceptance. Its long history since the first approval in 1988 further establishes its credibility and reliability in the treatment of fungal infections.
As a small molecule drug, Oxiconazole Nitrate offers advantages such as ease of formulation, administration, and cost-effectiveness. These characteristics contribute to its widespread use and availability in the market.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Oxiconazole Nitrate: fungal CYP51A1 inhibitors
From a biomedical perspective, fungal CYP51A1 inhibitors refer to substances or compounds that specifically target and inhibit the activity of the enzyme CYP51A1 in fungi. CYP51A1, also known as lanosterol 14-alpha-demethylase, is an essential enzyme involved in the biosynthesis of ergosterol, a crucial component of fungal cell membranes. By inhibiting CYP51A1, these inhibitors disrupt the synthesis of ergosterol, leading to impaired fungal cell growth and viability.
Fungal CYP51A1 inhibitors are of great interest in biomedicine as they serve as potential antifungal agents. By selectively targeting the fungal enzyme, these inhibitors can effectively combat fungal infections while minimizing adverse effects on human cells. They are commonly used in the development of antifungal drugs to treat various fungal infections, such as candidiasis, aspergillosis, and dermatophytosis.
Drug Target R&D Trends for Oxiconazole Nitrate
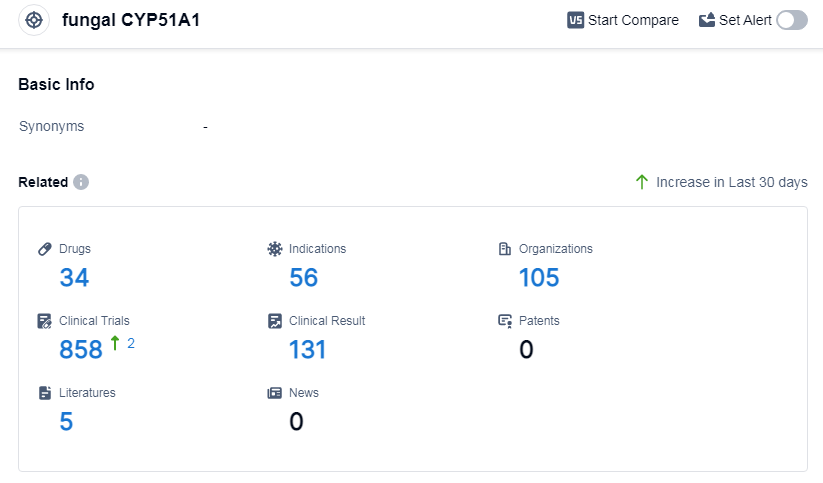
According to Patsnap Synapse, as of 13 Sep 2023, there are a total of 34 fungal CYP51A1 drugs worldwide, from 105 organizations, covering 56 indications, and conducting 858 clinical trials.
Based on the analysis of the provided data, the current competitive landscape of target fungal CYP51A1 is characterized by the involvement of multiple companies in the development of drugs targeting this enzyme. Bausch Health Cos., Inc., Pfizer Inc., and Merck & Co., Inc. are some of the companies with the highest number of drugs in the approved phase. The most approved indications for drugs targeting fungal CYP51A1 are mycoses, tinea pedis, and candidiasis. Small molecule drugs are progressing most rapidly under this target. China, the United States, and Japan are the countries/locations with the highest number of drugs in the approved phase.
The future development of target fungal CYP51A1 is promising, with ongoing research and development efforts by various companies and countries. Continued progress in China, as well as other countries, will contribute to the future development of drugs targeting fungal CYP51A1.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In conclusion, Oxiconazole Nitrate is a small molecule drug developed by Novartis Pharma AG. It targets fungal CYP51A1 and is indicated for the treatment of mycoses, tinea manus, tinea pedis, and tinea versicolor. With its highest phase of approval achieved globally, as well as its first approval in the United States in 1988, Oxiconazole Nitrate has established itself as a reliable and effective drug in the field of biomedicine.