Deep Scientific Insights on Pegvisomant's R&D Progress, Mechanism of Action, and Drug Target
Pegvisomant's R&D Progress
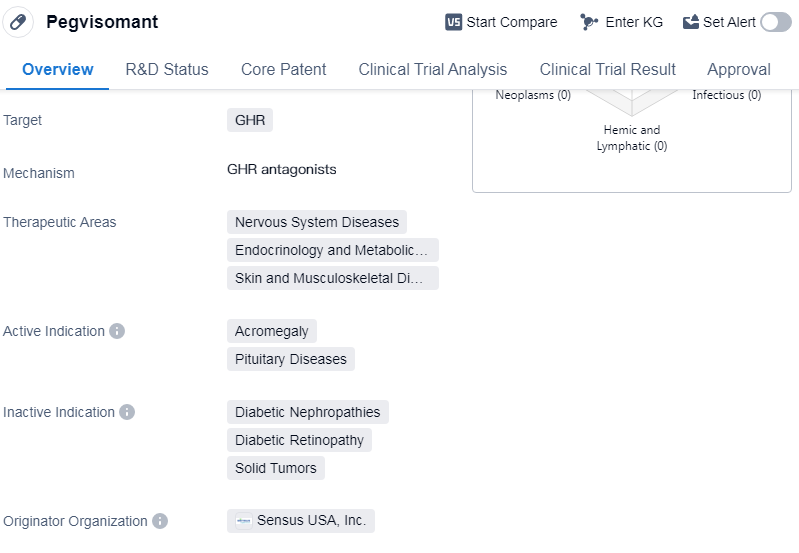
Pegvisomant is a hormone drug that targets the growth hormone receptor (GHR). It is primarily used in the treatment of various diseases related to the nervous system, endocrinology and metabolism, as well as skin and musculoskeletal disorders. The active indications for this drug include acromegaly and pituitary diseases.
Pegvisomant was first approved for use in the European Union in November 2002. It is important to note that Pegvisomant is classified as an orphan drug, which means it is intended to treat rare diseases or conditions that affect a small number of patients.
Pegvisomant was developed by Sensus USA, Inc., an originator organization specializing in the pharmaceutical industry. As of the latest available information, the drug has reached its highest phase of development, which is approved for use globally. This indicates that it has successfully completed clinical trials and met the necessary regulatory requirements for market authorization.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Pegvisomant: GHR antagonists
GHR antagonists are substances or compounds that inhibit the activity of the GHR, which is a protein found on the surface of cells that binds to growth hormone (GH) and initiates a signaling cascade leading to various physiological effects, including growth and metabolism regulation.
From a biomedical perspective, GHR antagonists can be used as therapeutic agents to modulate the effects of GH. By blocking the interaction between GH and its receptor, these antagonists can potentially reduce the effects of excessive GH signaling, such as in conditions like acromegaly or gigantism.
It is worth noting that GHR antagonists should be used with caution, as GH plays important roles in normal growth and development. Therefore, careful dosage and monitoring are necessary to avoid unwanted side effects or disruptions to normal physiological processes.
Drug Target R&D Trends for Pegvisomant
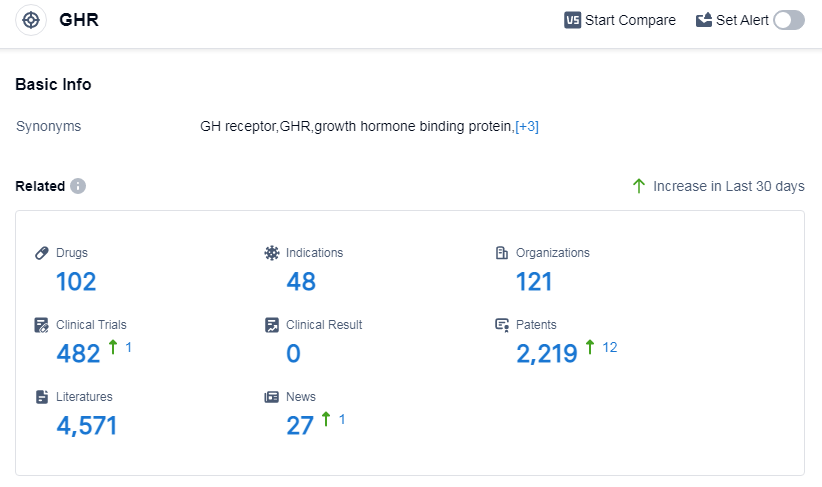
According to Patsnap Synapse, as of 13 Sep 2023, there are a total of 102 GHR drugs worldwide, from 121 organizations, covering 48 indications, and conducting 482 clinical trials.
The analysis of the target GHR reveals a competitive landscape with multiple companies actively involved in the development of drugs for growth-related disorders. Pfizer Inc., Novo Nordisk A/S, Changchun High & New Technology Industries (Group), Inc., and Anhui Anke Biotechnology (Group) Co., Ltd. are the leading companies in terms of R&D progress. Growth hormone deficiency is the most common indication for the target GHR, followed by other growth-related disorders. Hormone and biosimilar drugs are progressing rapidly, indicating intense competition in the market. China, the United States, Japan, and the European Union are the key countries/locations driving the development of drugs for the target GHR, with China showing significant progress. Overall, the target GHR presents a promising future for the pharmaceutical industry, with a focus on addressing various growth-related disorders and improving patient outcomes.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Pegvisomant is a hormone drug that targets the growth hormone receptor. It is primarily used in the treatment of acromegaly and pituitary diseases. The drug was first approved in the European Union in 2002 and is classified as an orphan drug. Developed by Sensus USA, Inc., Pegvisomant has reached its highest phase of development and is approved for use globally.