Levicept Reveals Promising Phase II Results for LEVI-04 in Moderate to Severe Osteoarthritis at ACR 2024
Levicept Ltd, a biotech firm dedicated to advancing LEVI-04, a pioneering therapy for osteoarthritis, is sharing findings from its encouraging Phase II study of LEVI-04 at the annual gathering of the American College of Rheumatology, known as ACR Convergence 2024. This event is scheduled to take place in Washington, DC, from November 14 to November 19, 2024. Initial results were revealed in August 2024.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
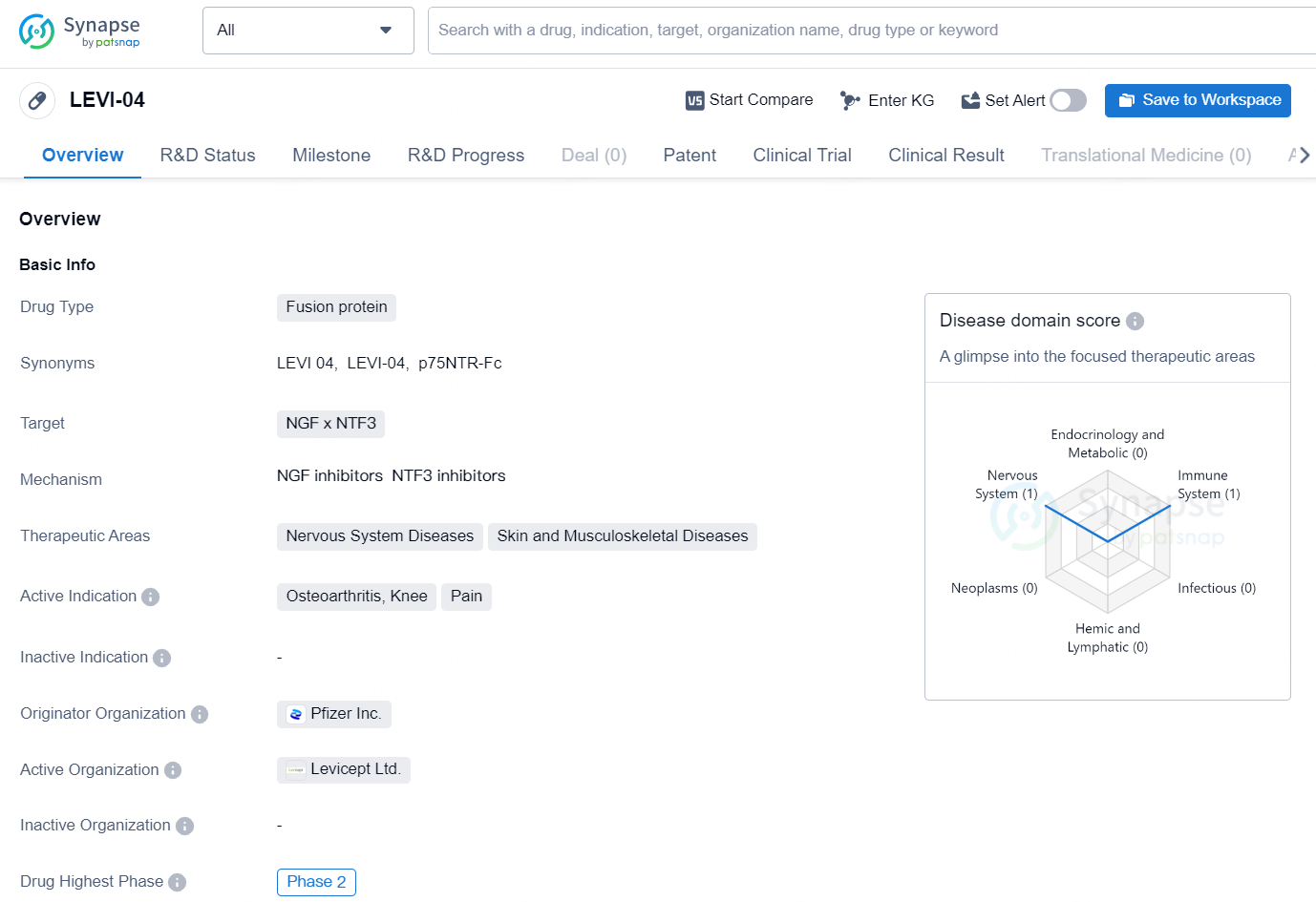
LEVI-04 is a specialized fusion protein of the p75 neurotrophin receptor (p75NTR-Fc) that alleviates pain by inhibiting NT-3 activity. It acts by supplementing the natural binding proteins for p75NTR and regulating the elevated levels of neurotrophins found in osteoarthritis.
The findings presented at the conference originate from Levicept’s Phase II study, which is a multiarm, multicenter, randomized, double-blind, placebo-controlled trial involving 518 subjects suffering from pain and disability due to osteoarthritis in the knee (ClinicalTrials.gov ID: NCT05618782).
LEVI-04 exhibited statistically significant improvements over placebo for the primary endpoint across all administered doses. The primary endpoint was the assessment of WOMACi pain, measured as the change from baseline at Week 17. The average decrease in WOMAC pain scores from baseline exceeded 50% for all three dosages of LEVI-04 (0.3 mg/kg, 1 mg/kg, 2 mg/kg), with all results being statistically significant compared to placebo (p<0.05 for all doses).
Over half of the patients treated with LEVI-04 experienced at least a 50% reduction in pain, while more than 35% reported a pain reduction of 70% or more by week 17. Secondary endpoints encompassed WOMAC subscales related to function and joint stiffness, overall patient assessments, and daily pain measures, all of which also showed statistically significant differences from placebo.
Standard safety evaluations, along with assessments of the peripheral nervous system, indicated that LEVI-04 was well tolerated. There was no noticeable increase in the occurrence of serious adverse events (SAEs), treatment-emergent adverse events (TEAEs), or joint pathologies such as rapidly progressive osteoarthritis compared to placebo, as determined by thorough radiographic analyses.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
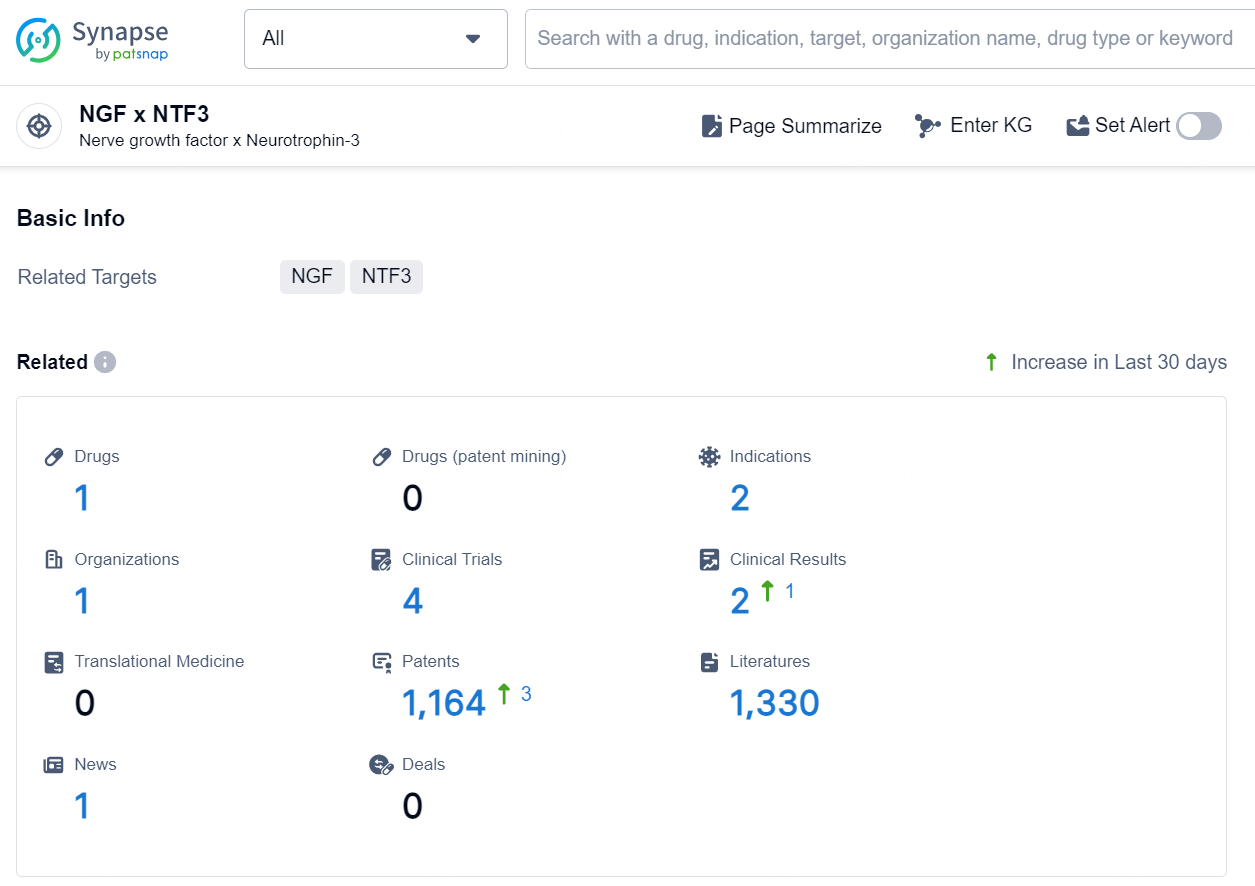
According to the data provided by the Synapse Database, As of November 19, 2024, there are 1 investigational drug for the NGF x NTF3 target, including 2 indications, 1 R&D institution involved, with related clinical trials reaching 4, and as many as 1164 patents.
LEVI-04 is a fusion protein drug developed by Pfizer Inc., targeting the NGF x NTF3 proteins. It falls under the therapeutic areas of Nervous System Diseases, Skin and Musculoskeletal Diseases, with an active indication for Osteoarthritis, Knee, and Pain. The drug has reached the highest global phase of Phase 2 in its development.