Exploring Sargramostim's Revolutionary R&D Successes and its Mechanism of Action on Drug Target
Sargramostim's R&D Progress
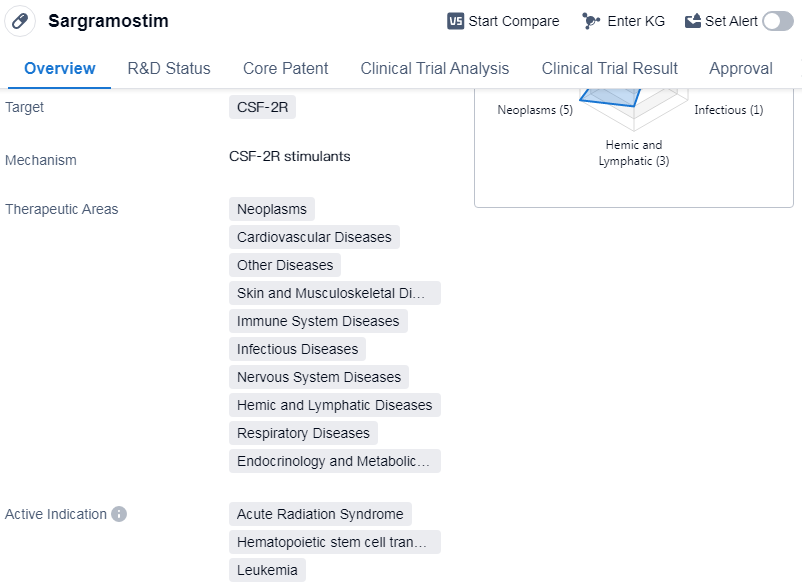
Sargramostim is a drug classified as a colony-stimulating factor, which means it stimulates the production of certain cells in the body. It specifically targets the CSF-2R receptor. The drug has been approved for use in various therapeutic areas, including neoplasms (abnormal growth of cells), cardiovascular diseases, skin and musculoskeletal diseases, immune system diseases, etc.
Sargramostim has shown efficacy in treating several conditions. It has been approved for use in acute radiation syndrome, a condition caused by exposure to high levels of radiation. The drug is also used in hematopoietic stem cell transplantation, a procedure where stem cells are transplanted to replace damaged or destroyed bone marrow. Additionally, Sargramostim is used in the treatment of leukemia, myeloid leukemia, primary graft dysfunction, melanoma, COVID-19, etc.
The drug was developed by Amgen, Inc., a prominent organization in the pharmaceutical industry. It received its first approval in the United States in March 1991. Sargramostim is regulated as an orphan drug, which means it is intended to treat rare diseases or conditions.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Sargramostim: CSF-2R stimulants
CSF-2R stimulants refer to substances or drugs that stimulate the CSF-2R receptor. CSF-2R stands for colony-stimulating factor-2 receptor, which is a protein found on the surface of certain cells in the immune system. CSF-2R is involved in the regulation and differentiation of immune cells, particularly granulocytes and macrophages.
Stimulating the CSF-2R receptor can enhance the production and function of these immune cells, leading to increased immune response and potentially improved defense against infections or diseases. CSF-2R stimulants can be used in various biomedical applications, such as immunotherapy, where they are designed to activate the immune system to target and eliminate cancer cells.
It's important to note that CSF-2R stimulants are a specific type of drug or substance that targets the CSF-2R receptor. They are not to be confused with other types of stimulants or drugs used for different purposes.
Drug Target R&D Trends for Sargramostim
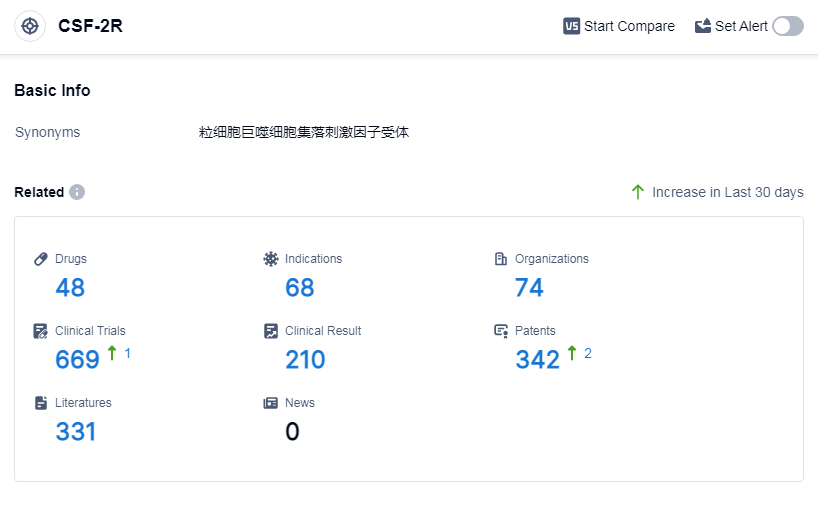
According to Patsnap Synapse, as of 8 Sep 2023, there are a total of 48 CSF-2R drugs worldwide, from 74 organizations, covering 68 indications, and conducting 669 clinical trials.
The analysis of the target CSF-2R reveals a competitive landscape with several companies actively developing drugs for various indications. Amgen, Inc., Partner Therapeutics, Inc., and Changchun High & New Technology Industries (Group), Inc. are the companies growing fastest under this target. Neutropenia, leukopenia, myelodysplastic syndromes, and melanoma are the most common indications for approved drugs. Colony-stimulating factors and biosimilars are the types of drug progressing most rapidly. China is the country developing fastest under the CSF-2R target, followed by the United States, India, and the European Union. The future development of CSF-2R will likely see continued competition among companies, with a focus on developing drugs for neutropenia and other relevant indications.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Sargramostim is a colony-stimulating factor drug that targets the CSF-2R receptor. It has been approved for use in various therapeutic areas, including neoplasms, cardiovascular diseases, and immune system diseases. The drug has shown efficacy in treating conditions such as acute radiation syndrome, hematopoietic stem cell transplantation, and various types of leukemia. Developed by Amgen, Inc., Sargramostim received its first approval in the United States in 1991 and is regulated as an orphan drug.