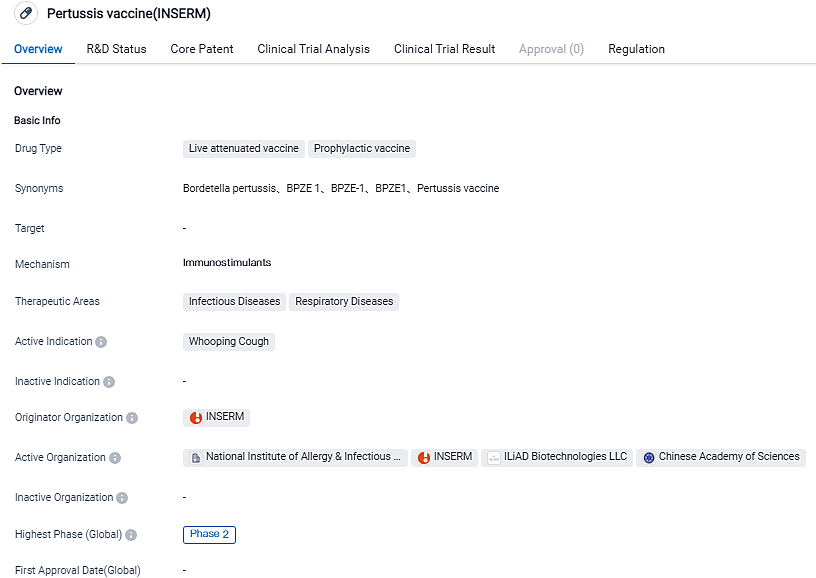
ILiAD Biotechnologies announces unique evidence of protection against B. pertussis infection in Phase 2b Human Challenge Trials with BPZE1 Vaccine
ILiAD Biotechnologies, LLC, a biotech firm in the clinical trial phase, which is spearheading the creation of an unparalleled, progressive pertussis vaccine, reports promising preliminary results from the CHAMPION-1 clinical trial.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
ILiAD Biotechnologies announced today that its BPZE1 intranasal vaccine for pertussis has successfully achieved the main goal of providing protection against nasopharyngeal B.pertussis colonization as per the Phase 2b CHAMPION-1 trial. Most individuals receiving BPZE1 had either no or mild systemic and nasal/respiratory symptoms of limited duration, and no serious adverse events were reported.
The CHAMPION-1 Phase 2b Human Challenge trial is an organized, double-blind, placebo-controlled test of the BPZE1 vaccine with healthy adults as subjects. The trial's target was to show that early immunization with BPZE1 safeguards against colonization, as shown by negative B.pertussis culture occurring after a harsh B.pertussis encounter 2-4 months post-vaccination.
Leading investigator Prof. Robert Read, holder of the Chair of Infectious Diseases at the University of Southampton and presiding over the CHAMPION-1 trial commented, "This testing provided the first-ever proof of a pertussis vaccine successfully preventing B.pertussis colonization in a human challenge pattern. BPZE1’s capability to stimulate sterilizing mucosal immunity and to prevent B.pertussis colonization in the majority of participants post-challenge is a remarkable achievement.”
Outcome signals that the BPZE1 vaccine does prevent B.pertussis colonization, as shown by both the key endpoint of the rate of participants with no colonization after the challenge in the per-protocol adequate inoculum group, and the noticeable reduction in the overall B.pertussis bacterial load.
Commenting on the results, ILiAD Chief Medical Officer, Stephanie Noviello, MD, stated, “This testing was meant to define the principal endpoint, test parameters, and sample volume required to support a successful Phase 3B. pertussis human challenge trial, our confidence in attaining this has now increased. The key data from the CHAMPION-1 clinical test will be announced at the World Vaccine Congress in Barcelona on October 19th.”
Pertussis is a dangerous ailment brought about by the extremely infectious respiratory bacterium Bordetella pertussis. It impacts nearly 16 million individuals worldwide each year, causing about 200,000 fatalities. Despite a global vaccination coverage of 84%, current vaccines have not been able to halt epidemics. Furthermore, present vaccines do not completely safeguard infants younger than 6 months, as the immunization process requires multiple injections typically at 2, 4 and 6 months.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
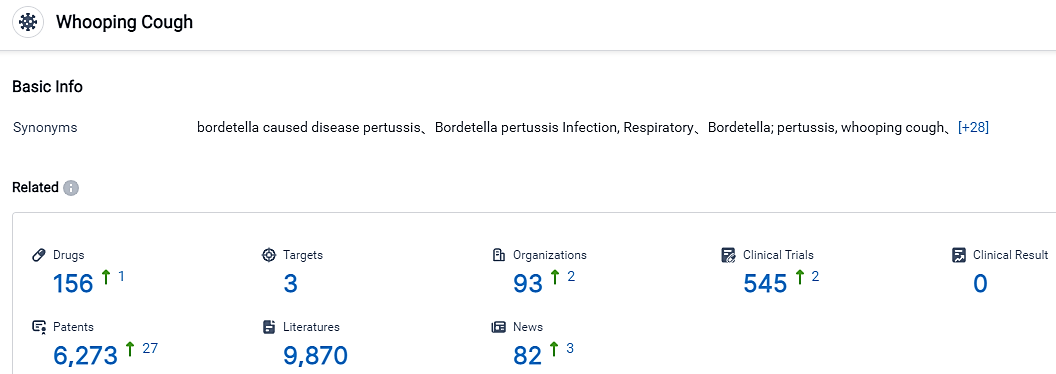
According to the data provided by the Synapse Database, As of September 7, 2023, there are 156 investigational drugs for Whooping Cough, including 3 targets, 93 R&D institutions involved, with related clinical trials reaching 545, and as many as 6273 patents.
China National Pharmaceutical Group Co., Ltd., Sanofi, and GSK Plc are the leading companies in terms of drug development. The targets "50S subunit," "30S subunit," and "TLR9" have been approved for drugs under the indication of Whooping Cough, highlighting their importance in the development of effective treatments. Prophylactic vaccines and combination vaccines are the most rapidly progressing drug types, indicating a strong focus on preventive measures and combination approaches in vaccination.