Anti-emetic Agent - 5-HT3 Antagonist
Nausea and vomiting are the most common adverse events after antitumor drug therapy and surgery. According to statistics, more than 90% of patients who receive highly emetic chemotherapy experience episodes of vomiting, and the incidence of postoperative nausea and vomiting (PONV) is 30% among general surgical patients, reaching up to 80% in high-risk populations. Episodes of vomiting can lead to dehydration, metabolic imbalance, degradation in self-care abilities, nutritional deficiencies, declines in mental and physical condition, wound dehiscence, esophageal mucosal lacerations, and more, all of which significantly affect the patient's quality of life, reducing the compliance with subsequent antitumor treatments and increasing postoperative complications and treatment costs.
Vomiting is the result of a multi-step reflex pathway controlled by the brain. Research shows that chemotherapy drugs can stimulate the release of 5-hydroxytryptamine (5-HT) in the body, and through the mediation of 5-HT3 receptor, transmit signals to the vomiting center, thereby causing vomiting.
5-HT3 receptor antagonists cannot reduce the production and release of 5-HT. Instead, they relieve the patient's vomiting symptoms by competitively blocking the binding of gastrointestinally released serotonin (5-hydroxytryptamine) to the 5-HT3 receptor, which disrupts the transmission of the excitatory chemoreceptor to the vomiting center. Clinically, it is widely used to prevent and treat nausea and vomiting caused by cancer radiotherapy and chemotherapy, anesthesia, and surgery.
5-HT3 receptor Competitive Landscape
According to the data provided by Patsnap Synapse-Global Drug Intelligence Database: the following figure shows that as of 4 Sep 2023, there are a total of 106 5-HT3 receptor drugs worldwide, from 134 organizations, covering 69 indications, and conducting 1947 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
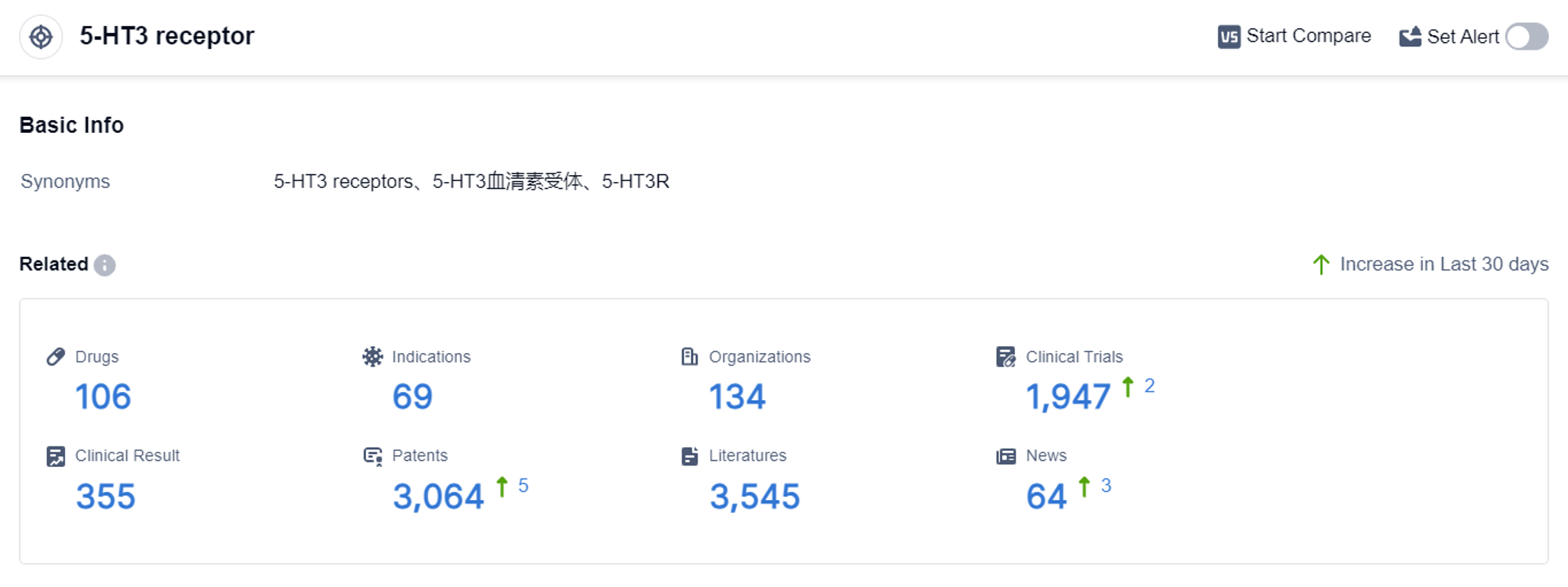
The analysis of the target 5-HT3 receptor reveals a competitive landscape with multiple companies actively developing drugs. The highest stage of development is the approved phase, indicating successful clinical trials and regulatory approval for marketing.
The indications for approved drugs include nausea, vomiting, chemotherapy-induced nausea and vomiting, and postoperative nausea and vomiting, among others. Small molecule drugs dominate the market, with herbal medicine also showing potential.
China is emerging as a significant player in the development of drugs targeting the 5-HT3 receptor. Overall, the target 5-HT3 receptor presents opportunities for innovation and competition in the pharmaceutical industry.
The first marketed highly selective 5-HT3 receptor antagonist——Ondansetron Hydrochloride
Ondansetron Hydrochloride is a small molecule drug that targets the 5-HT3 receptor. The drug is indicated for the management of nausea and vomiting caused by various conditions such as radiation-induced nausea and vomiting, chemotherapy-induced nausea and vomiting, postoperative nausea and vomiting, alcohol use disorder, alcoholism, and syncope vasovagal.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
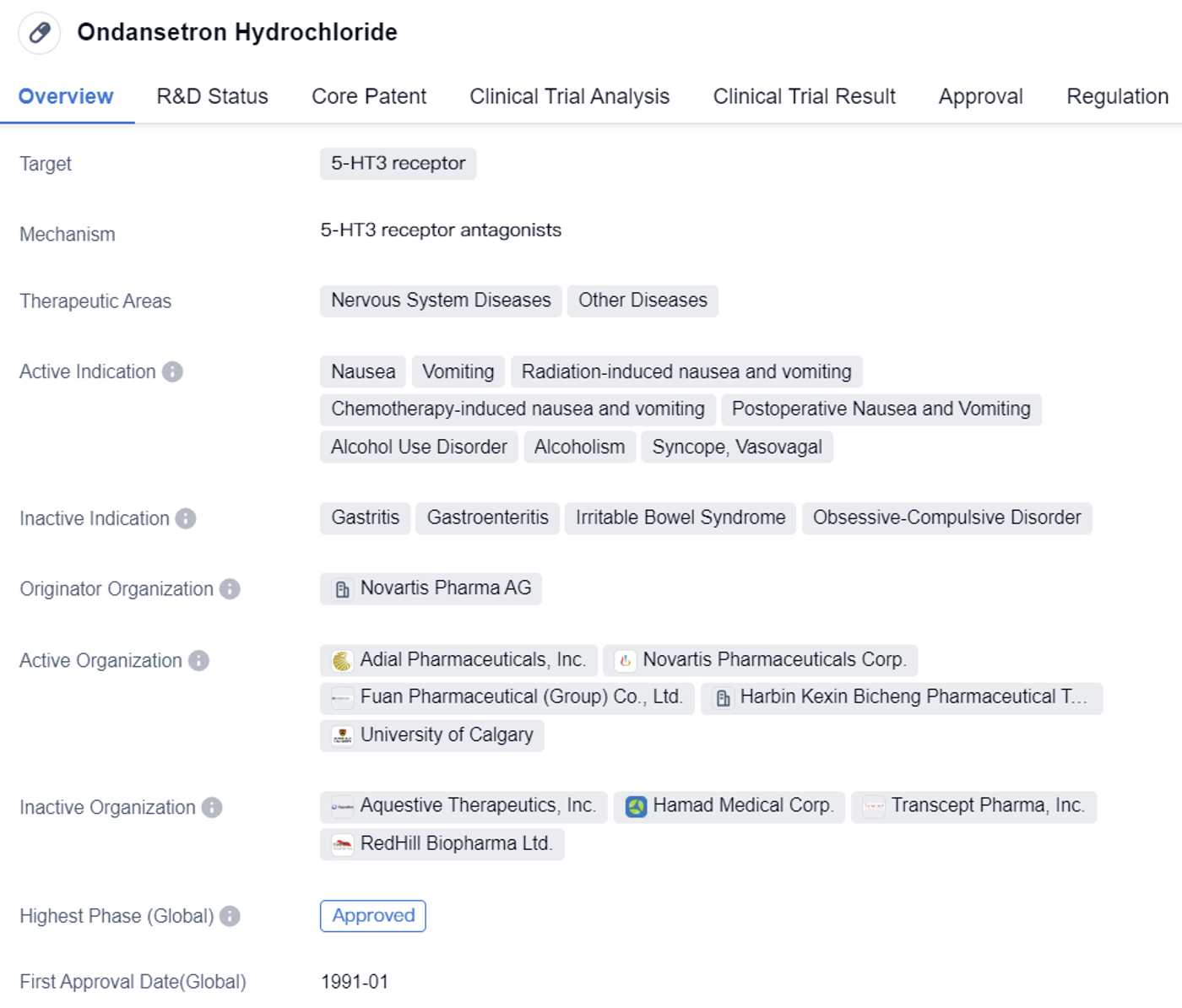
The drug was developed by Novartis Pharma AG, a renowned pharmaceutical company. It received its first approval in the United States in January 1991, making it a well-established drug in the market. Ondansetron Hydrochloride has also obtained approvals in other countries, indicating its global recognition and acceptance.
Ondansetron Hydrochloride's approval in both the global and Chinese markets highlights its efficacy and safety profile. The drug has successfully completed clinical trials and met the necessary regulatory requirements to be deemed safe and effective for patient use.
Overall, Ondansetron Hydrochloride is a well-established small molecule drug that effectively targets the 5-HT3 receptor. Its approval in multiple countries, including the United States and China, demonstrates its global recognition and acceptance. With its broad therapeutic indications and priority review status, the drug offers a valuable treatment option for patients suffering from various conditions associated with nausea and vomiting.
Second Generation 5-HT3 Receptor Antagonist——Palonosetron hydrochloride
Palonosetron hydrochloride is a small molecule drug that acts as a selective antagonist of the 5-HT3 receptor. It is primarily used in the treatment of nausea and vomiting associated with various conditions such as postoperative nausea and vomiting, chemotherapy-induced nausea and vomiting, and general vomiting. The drug was first approved in the United States in July 2003 and has since gained approval in other countries as well.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
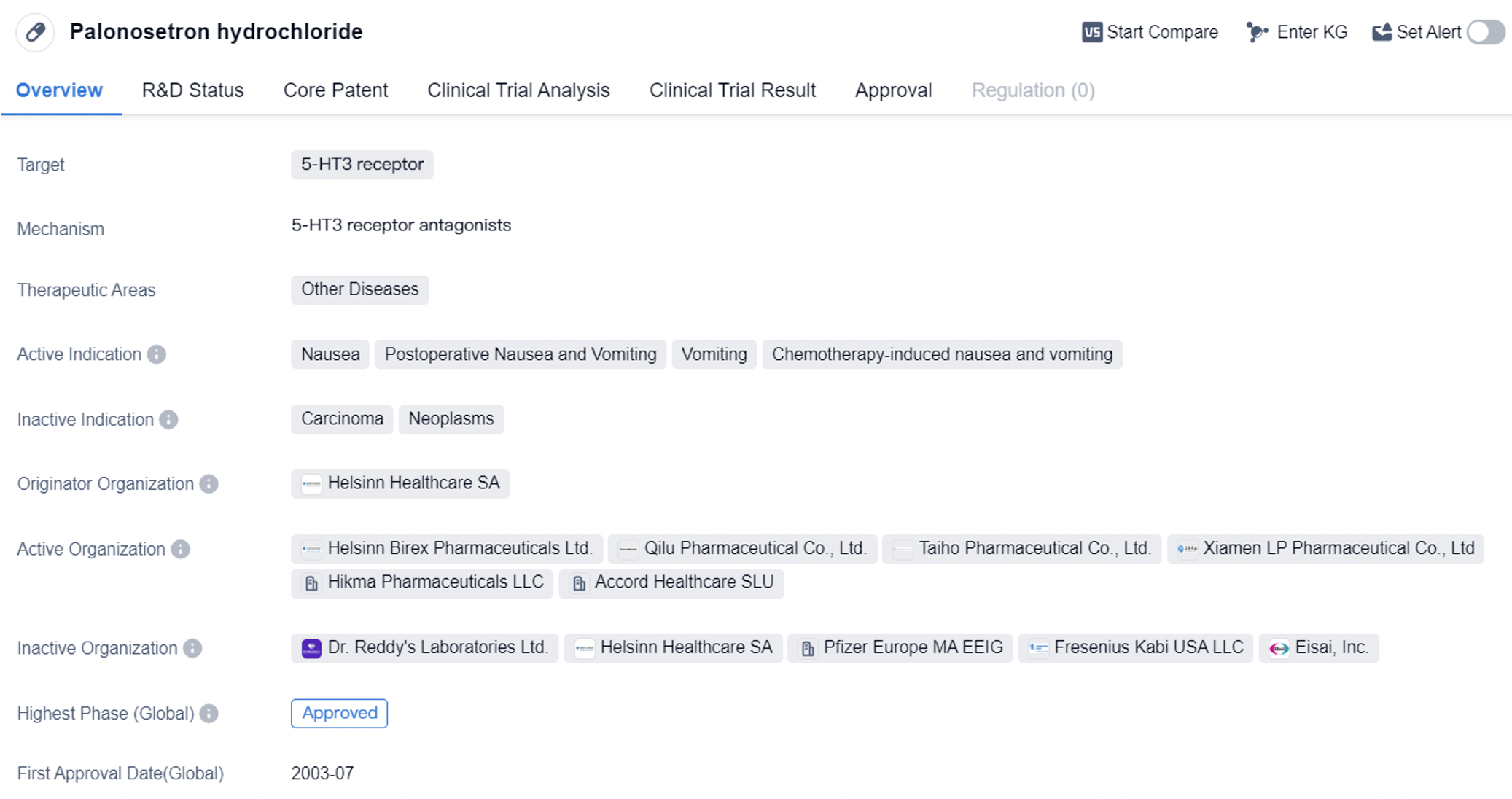
The approval of Palonosetron hydrochloride in multiple countries, including the United States and China, highlights its global significance and potential market reach. The drug's approval in these key markets indicates its safety and efficacy profile, as well as its potential to address unmet medical needs in the treatment of nausea and vomiting.
The therapeutic effects among the first generation of 5-HT3 receptor antagonists have no significant differences. Compared to the first generation of 5-HT3 receptor antagonists, the second generation Palonosetron hydrochloride has better efficacy in preventing acute and delayed vomiting caused by highly emetogenic and moderately emetogenic chemotherapeutic drugs.
Overall, Palonosetron hydrochloride is a small molecule drug that targets the 5-HT3 receptor and is primarily used to alleviate nausea and vomiting. Its approval in various countries, including the United States and China, underscores its potential as a therapeutic option for patients suffering from these symptoms.