Novo Nordisk Expands Wegovy Rollout in Europe Amid Supply Chain Challenges
Novo Nordisk is vigorously pursuing the rollout of the obesity medication, Wegovy, across Europe, despite grappling with supply chain issues. The Denmark-based pharmaceutical company is striving to persuade European administrative bodies and insurance firms to cover the cost of the drug, as it seeks to establish it as more than just a wellness prescription.
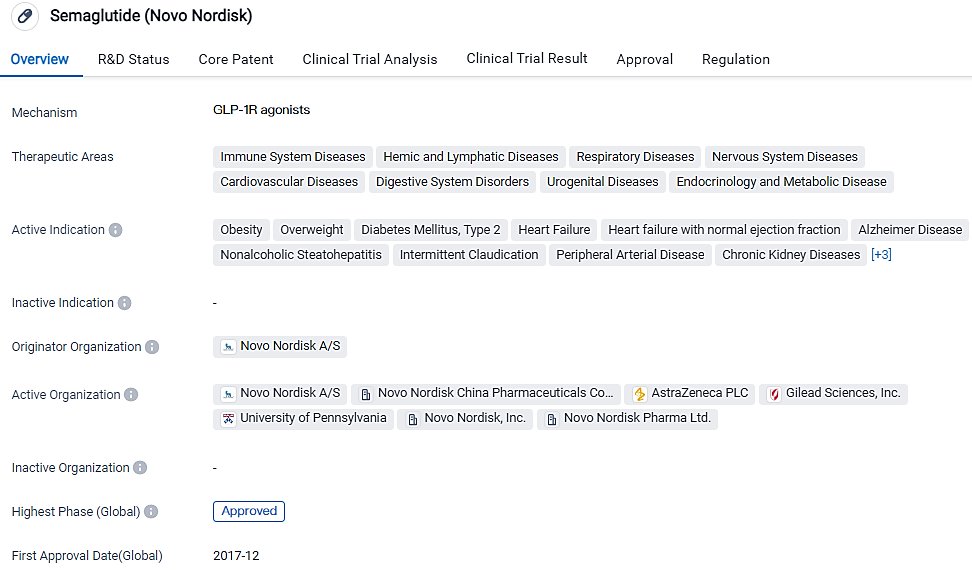
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Here are the nations where Wegovy has been released so far:
UNITED STATES
Wegovy entered the U.S. market in June 2021, however, because of an initial production problem, it wasn't until December 2022 that Novo was able to make all doses available there.
The four-week treatment plan is listed at roughly $1,350, regardless of the dosage, without considering any possible discounts or refunds.
The weekly treatment begins with a dose of 0.25 milligrams of the active ingredient, semaglutide, and is gradually increased up to the stable dose of 2.4 milligrams.
DENMARK
In early 2023, Wegovy was introduced in Denmark in all dosage forms. Approximately 2,370.60 Danish crowns is the cost for a four-week supply of the maintenance dosage.
Denmark's state-provided healthcare doesn't provide reimbursement for weight-loss medications, and the largest private health insurer in the country, which covers about half of the population, will end its reimbursement of weight-loss drugs from January the following year due to overwhelming demand.
According to the Danish Minister for Health, reimbursement for Wegovy could cost the government a sum close to $4 billion annually.
NORWAY
Wegovy was released in Norway with all dosage levels early in 2023. The maintenance dosing's four-week supply has a price of 2,775.30 Norwegian crowns.
In January, the Norwegian Medicines Agency announced that it would not subsidise the medication for weight loss treatment, stating the pricing was too steep compared to its proven health benefits.
GERMANY
Wegovy was introduced to the German market on July 29, with a price tag of 301.91 euros for a four week supply at the maintenance dosage. A four-week starter dosage is priced at 171.92 euros.
Governmental health insurance plans will not cover Wegovy's cost for an estimated 90% of Germans, according to a legislation that prevents them from covering weight-loss treatments.
For the remaining 10% who privately insure themselves, the coverage varies. Allianz, an insurance company, stated it would cover the cost of the drug if it was medically necessary, while Debeka confirmed their plans do not include weight-loss treatments.
UNITED KINGDOM
On September 4, Wegovy was made accessible in the UK via what Novo described as a "constrained and measured introduction". The company has not yet disclosed the price or the supply volume.
Simple Online Pharmacy, an internet-based pharmaceutical chain operating out of the UK, states it will retail Wegovy for a price between 199 pounds and 299 pounds, inclusive of a general practitioner consultation, prescription and dispensing costs.
Novo announced that the drug would be available within the framework of the National Health Service's weight management programme and also "privately via a certified healthcare provider".
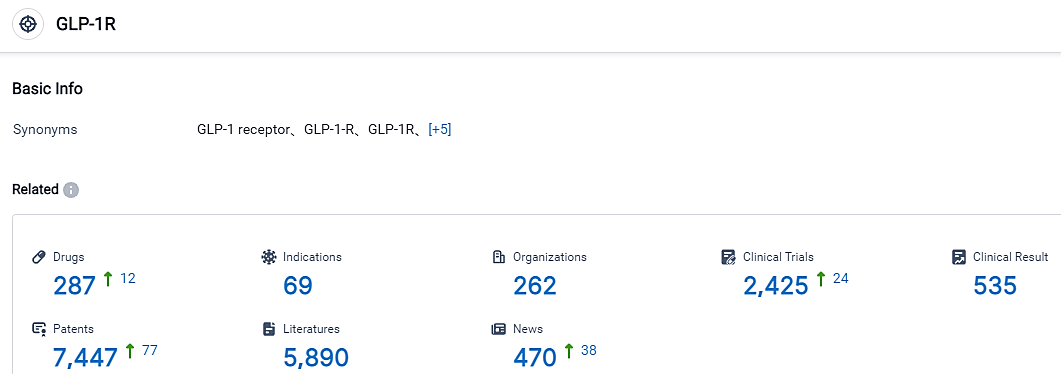
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of September 7, 2023, there are 287 investigational drugs for the GLP-1R target, including 69 applicable indications, 262 R&D institutions involved, with related clinical trials reaching 2425, and as many as 7447 patents.
The pharmaceutical sector views the field of obesity as highly competitive. InQpharm Group, Novo Nordisk A/S, Pfizer Inc., and Sanofi are amongst the top companies leading the progression and quantity of drug production. GLP-1R stands out as the primary receptor target among all verified drugs, revealing its crucial role in obesity treatment. The condition of obesity, therefore, offers considerable scope for further scientific exploration and enhancement.