Exploring Zanubrutinib: Advances in BTK Inhibition and Broad Therapeutic Applications
Zanubrutinib is a small molecule drug that targets the BTK enzyme and is approved for use in various therapeutic areas including neoplasms, immune system diseases, cardiovascular diseases, hemic and lymphatic diseases, urogenital diseases, nervous system diseases, skin and musculoskeletal diseases, and respiratory diseases.
The drug was developed by BeiGene Ltd. and has received approval at the highest phase in both the global and Chinese markets. Its first approval was in the United States in November 2019. Zanubrutinib has been granted various regulatory designations including Priority Review, Conditional marketing approval, Accelerated Approval, Fast Track, Orphan Drug, Breakthrough Therapy, and Special Review Project.
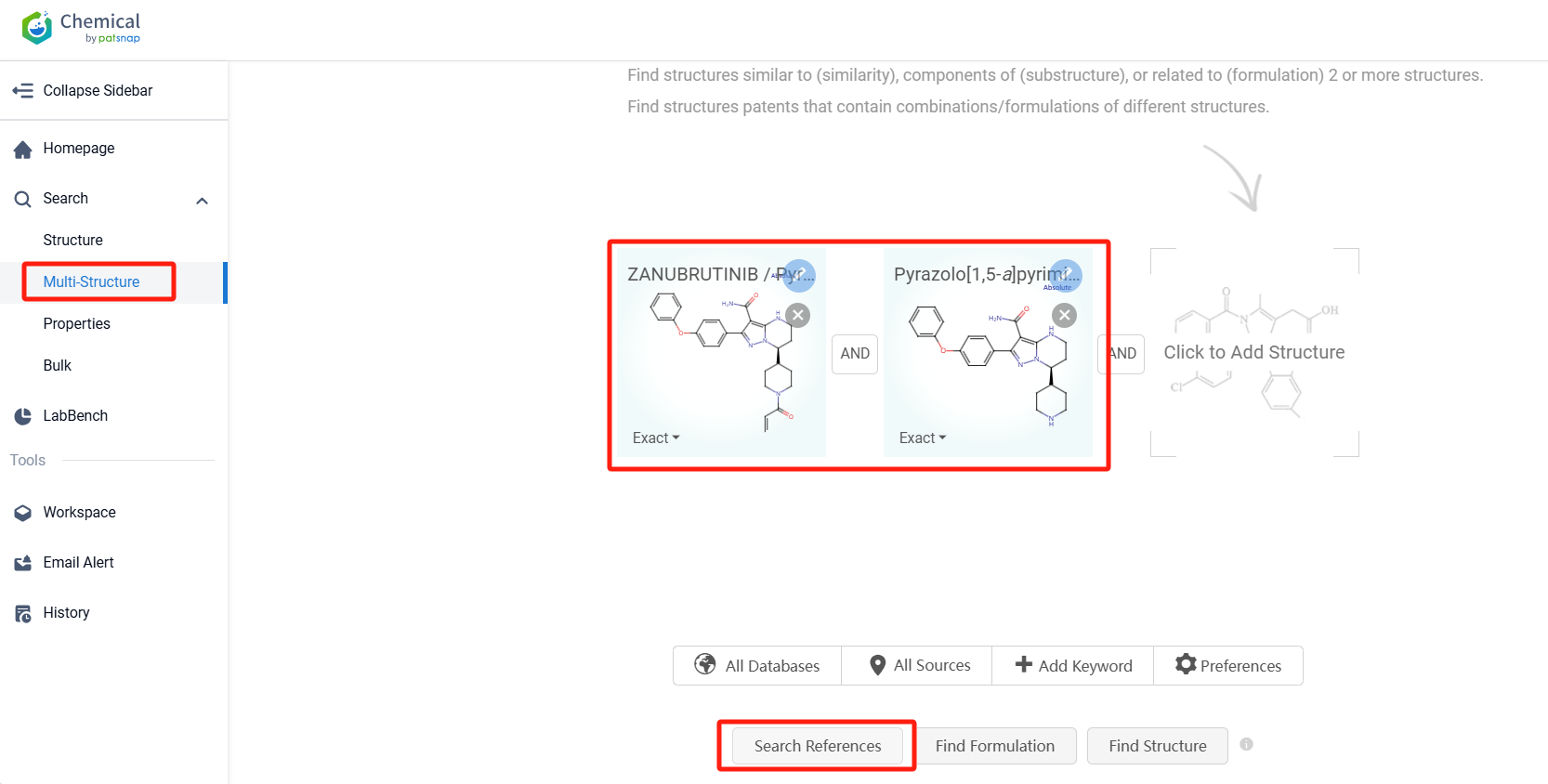
Log in to the Patsnap Chemical. Select the Multi-Structure search, and search for the reactants and products in the specific steps of the synthesis route of Zanubrutinib. click on search references, and you can query the relevant literature.
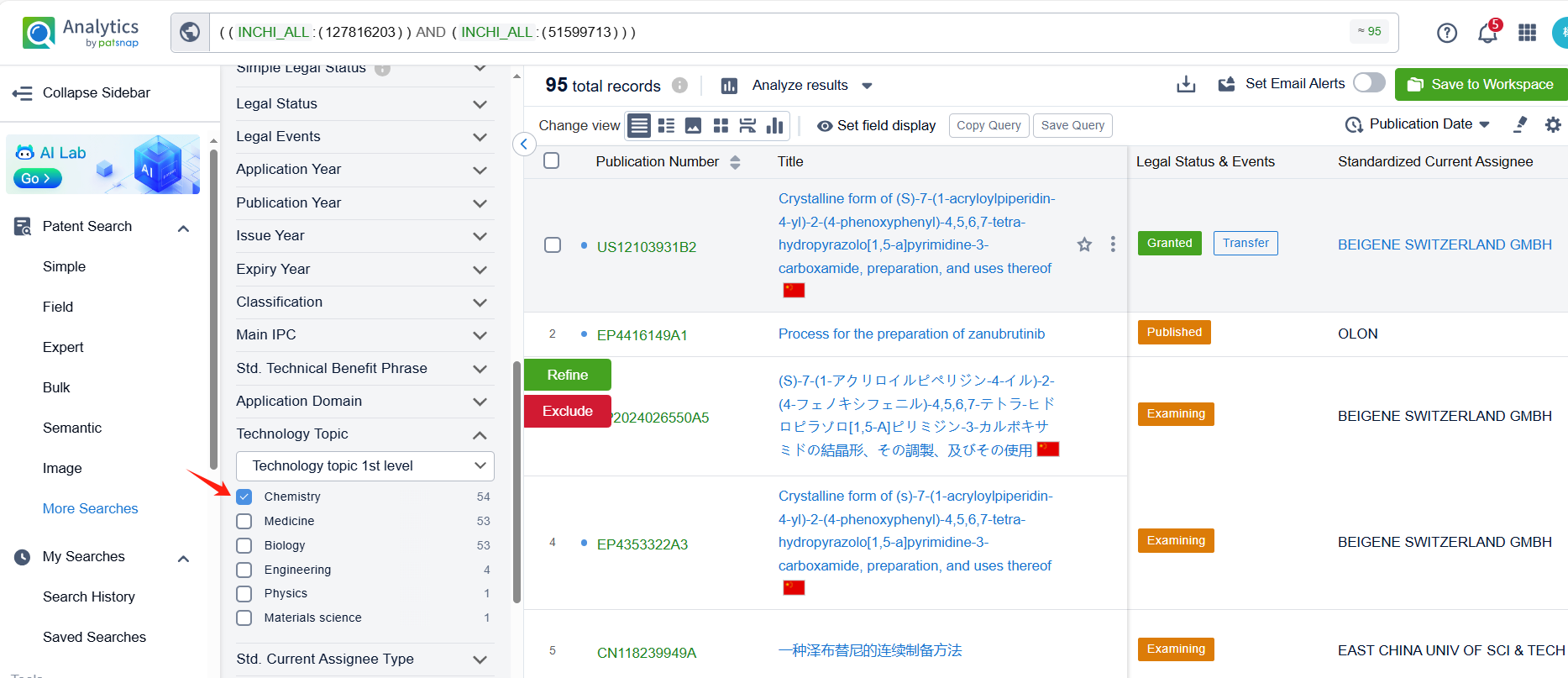
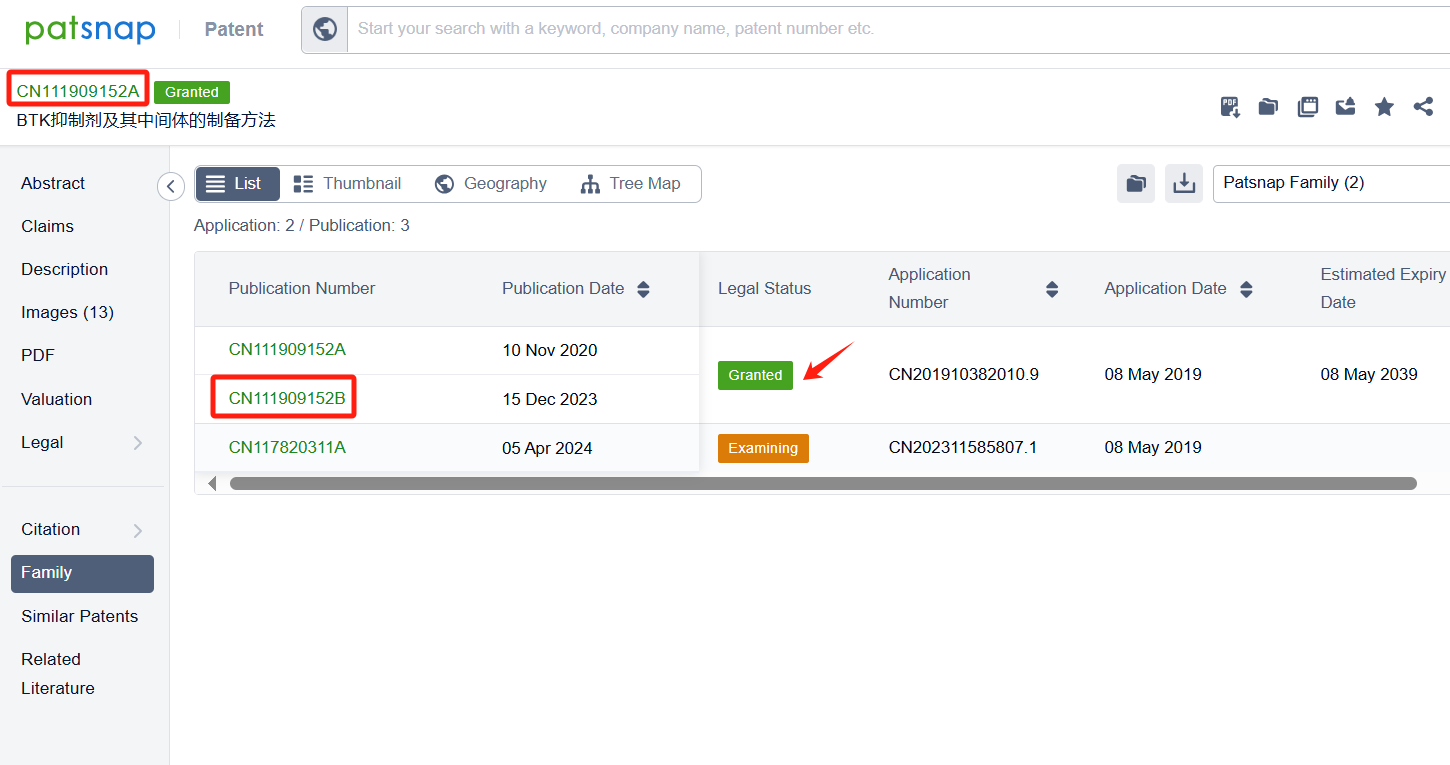
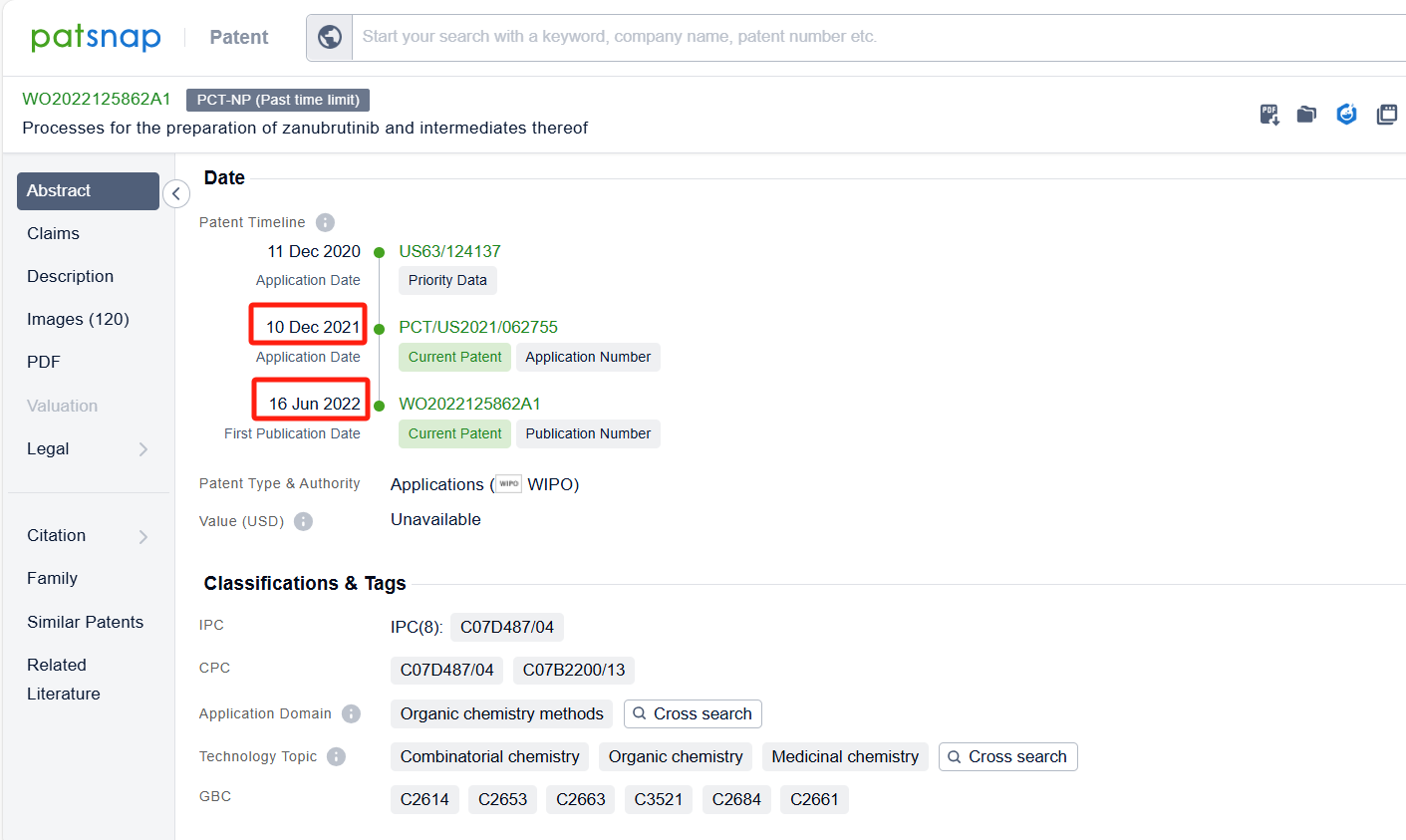
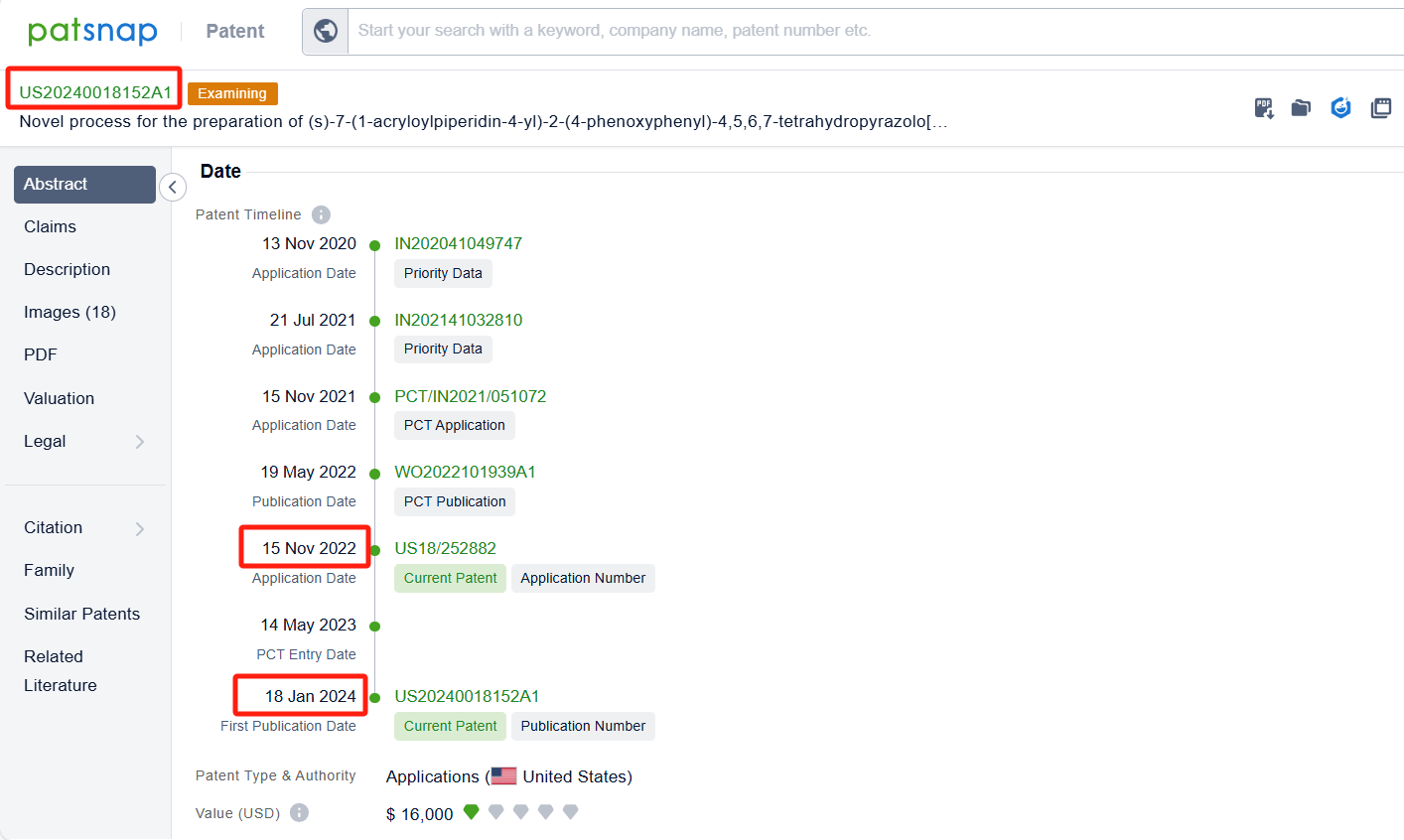
Linked to the Patsnap patent, check the 'Chemistry' category under the technical subject classification and click on the filter, which will allow you to accurately retrieve the design and improvement of the Zanubrutinib process route by BeiGene (Suzhou) Co. Ltd. and other companies,Such as BeiGene (Suzhou) Co. Ltd.'s patent CN111909152A (application date 20190508, publication date 20201110) describes a method for preparing Zanubrutinib, The present invention is to significantly improve the reaction yield and product purity by optimizing reaction parameters, especially by improving the reaction temperature in the step of synthesizing intermediate BG-11A from intermediate BG-10. The patent was granted on December 15, 2023.Teva Pharmaceuticals International GmbH's international patent WO2022125862A1(application date 20211210, publication date 20220616) is to improve the process for synthesizing Zanubrutinib, a Bruton's tyrosine kinase inhibitor, with high yields, high chemical purity, and high optical purity, without the need for multiple chiral purification steps and multiple chromatographic purification steps. Additionally, MSN Laboratories Pvt Ltd.'s patent US20240018152A1 is to provide a simple, cost-effective, viable, environment friendly, and commercially scalable process for the preparation of Zanubrutinib and its intermediates, which results in yield improvement.
In summary, Zanubrutinib is a small molecule drug targeting the BTK enzyme that has been approved for the treatment of a wide range of therapeutic areas and active indications. It has received approvals in both the global and Chinese markets and has been granted multiple regulatory designations, reflecting its potential to meet significant unmet medical needs in the pharmaceutical industry.
AI built to maximize IP and R&D efficiency
Redefine chemical FTO with a range of structure retrieval options at your fingertips, from exact matches to similarity searches, all powered by deep data processing techniques and proprietary AI algorithms to eliminate the risk of omitting key results.