FDA Approves Syndax's Revumenib: First Menin Inhibitor for Relapsed Acute Leukemia with KMT2A Translocation
Syndax Pharmaceuticals (Nasdaq: SNDX) announced that the U.S. Food and Drug Administration (FDA) has granted approval for Revuforj® (revumenib), marking it as the first and sole menin inhibitor indicated for treating relapsed or refractory (R/R) acute leukemia associated with a lysine methyltransferase 2A gene (KMT2A) translocation in adults and children aged one year and older. The FDA had previously awarded Revuforj Breakthrough Therapy and Fast Track designations, as well as Priority Review. The New Drug Application (NDA) received approval via the FDA’s Real Time Oncology Review (RTOR) initiative.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
"The authorization of Revuforj marks a significant milestone that showcases the commitment and perseverance of all those involved, particularly the patients and healthcare professionals engaged in our study, along with our skilled Syndax team," stated Michael A. Metzger, Chief Executive Officer of Syndax. "We are ready to introduce Revuforj this month and pledge to expedite its development throughout the treatment spectrum for KMT2A-rearranged acute leukemias and mutant NPM1 AML."
The effectiveness of Revuforj was assessed through an FDA analysis involving 104 patients suffering from R/R acute leukemia with a KMT2A translocation who received Revuforj during the Phase 1/2 AUGMENT-101 trial. Among the efficacy cohort, the combined rate of complete remission (CR) and CR with partial hematological recovery (CRh) was 21% (22 out of 104 patients; 95% CI: 13.8%, 30.3%). The median duration of CR+CRh was 6.4 months (95% CI: 2.7, not estimable), and the median time to achieve CR or CRh was 1.9 months (ranging from 0.9 to 5.6 months). Following treatment with Revuforj, 23% (24 out of 104 patients) underwent hematopoietic stem cell transplantation (HSCT). The findings from the 104-patient efficacy assessment align with earlier published results from a protocol-defined interim analysis of Phase 2 patients with R/R KMT2Ar acute leukemia in the AUGMENT-101 trial (n=57) published in the Journal of Clinical Oncology.
"The FDA's sanctioning of the initial menin inhibitor is a significant advancement for patients with R/R acute leukemia featuring a KMT2A translocation, which is linked to extremely poor prognosis," commented Ghayas C. Issa, M.D., Associate Professor of Leukemia at The University of Texas MD Anderson Cancer Center. "The notable clinical advantage and strong efficacy demonstrated by Revuforj illustrate a major enhancement over historically available treatments for these patients, representing a promising new treatment avenue."
The safety profile of Revuforj was evaluated based on an FDA review of 135 patients with R/R acute leukemia having a KMT2A translocation who were administered Revuforj. The most prevalent adverse reactions (≥20%), including laboratory anomalies, comprised hemorrhage, nausea, elevated phosphate levels, musculoskeletal discomfort, infections, increased aspartate aminotransferase, febrile neutropenia, elevated alanine aminotransferase, increased intact parathyroid hormone, bacterial infections, diarrhea, differentiation syndrome, prolonged QT interval on electrocardiogram, diminished phosphate levels, elevated triglycerides, reduced potassium levels, appetite loss, constipation, edema, viral infections, fatigue, and increased alkaline phosphatase. The occurrence of adverse reactions that necessitated dose modification or led to permanent discontinuation was relatively low at 10% and 12% of patients, respectively.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
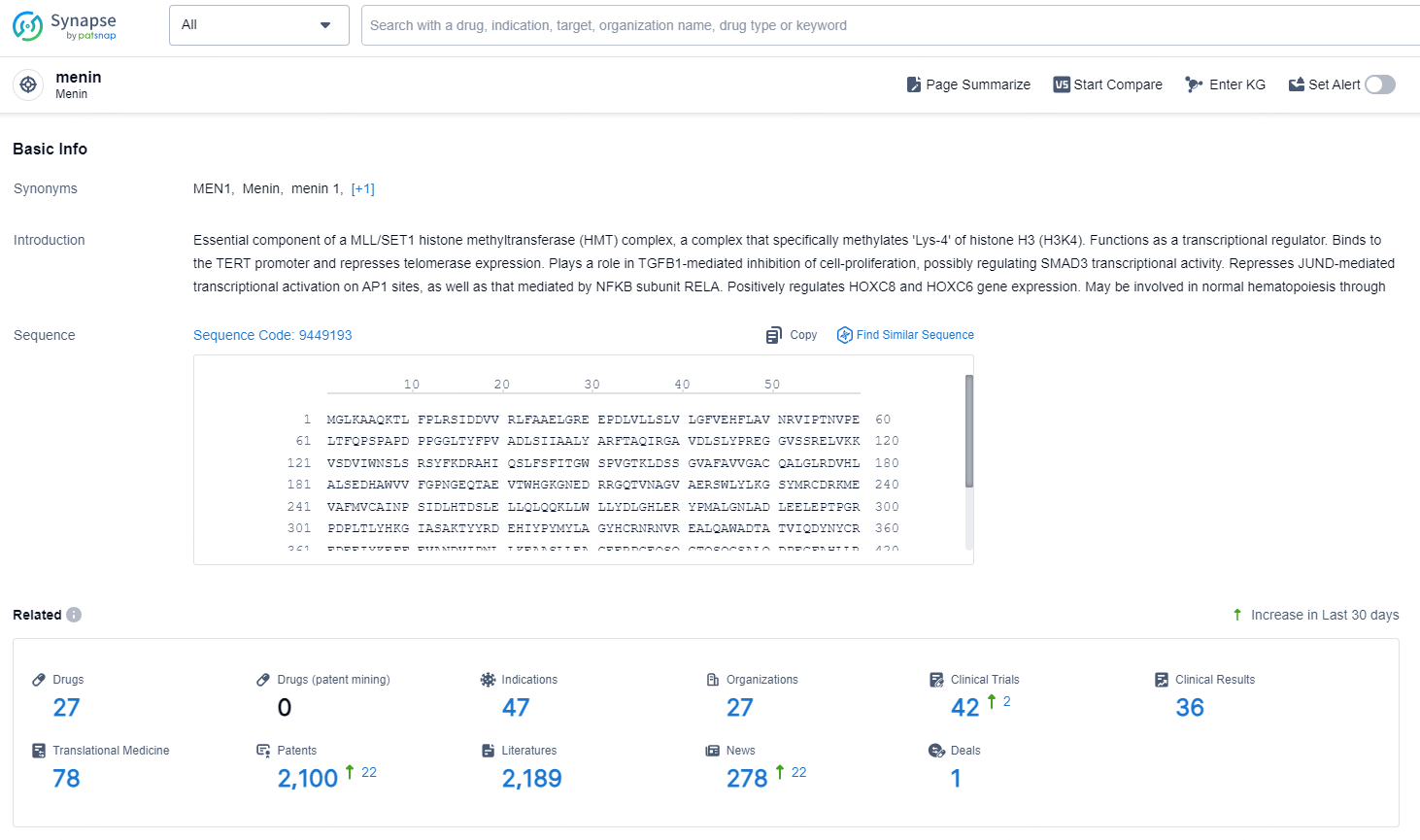
According to the data provided by the Synapse Chemical, As of November 19, 2024, there are 27 investigational drugs for the menin target, including 47 indications, 27 R&D institutions involved, with related clinical trials reaching 42, and as many as 2100 patents.
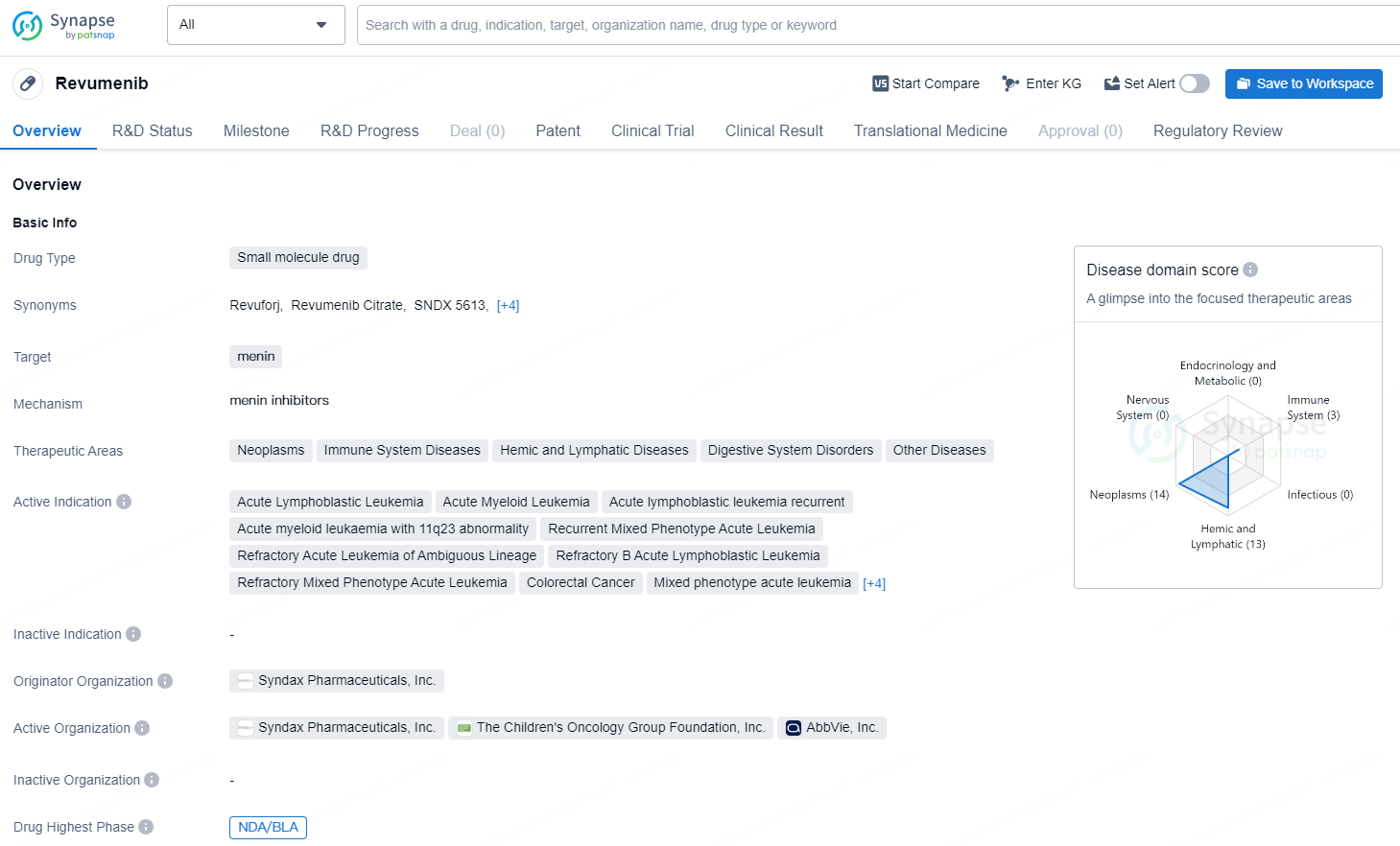
Revumenib is a small molecule drug developed by Syndax Pharmaceuticals, Inc. The drug targets menin and is currently in the highest phase of development, NDA/BLA, on a global scale. It is indicated for various therapeutic areas including neoplasms, immune system diseases, hemic and lymphatic diseases, digestive system disorders, and other diseases.