FDA Approves Acepodia's ACE1831 for IgG4-Related Disease Trial
Acepodia (6976:TT), a biotechnology firm in the clinical stage that focuses on pioneering cell therapies through its distinctive Antibody-Cell Conjugation (ACC) and allogeneic gamma delta 2 (γδ2) T cell technology platforms, announced the receipt of U.S. Food and Drug Administration (FDA) approval for its Investigational New Drug (IND) application for its primary candidate, ACE 1831, addressing IgG4-related disease (IgG4-RD), an autoimmune disorder characterized by multi-organ involvement and fibro-inflammatory processes.
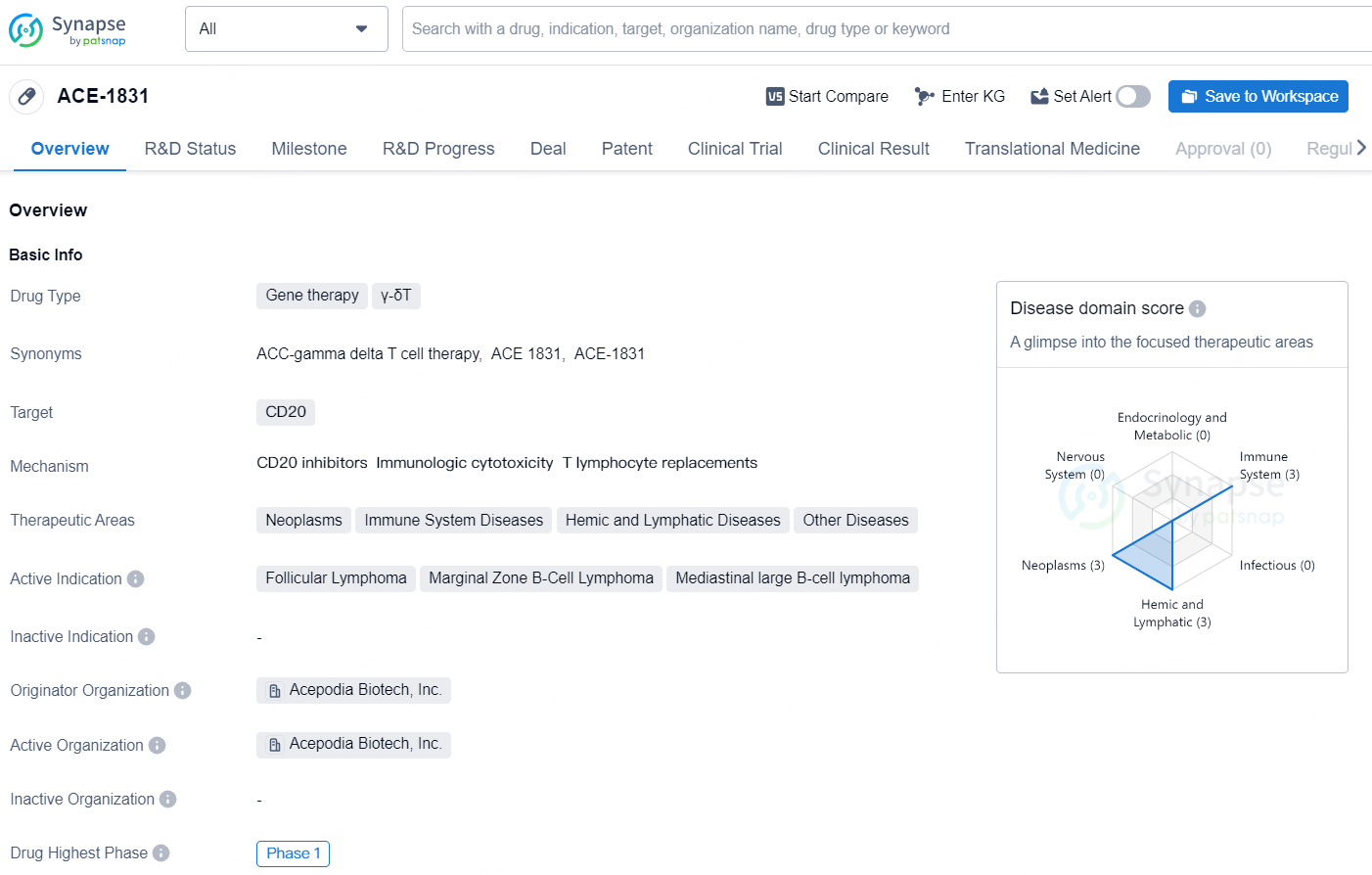
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
The IND approval enables Acepodia to explore the safety and effectiveness of ACE1831 in individuals with IgG4-RD, marking its initial foray into autoimmune conditions. ACE1831 employs bioorthogonal chemistry to conjugate CD20-targeting antibodies with gamma delta T cells, resulting in a readily available, non-genetically modified T cell therapy that can be produced more easily and may reduce the risk of side effects commonly associated with autologous CAR-T cell therapies, such as malignancies in T cells. Additionally, ACE1831 is currently undergoing a Phase 1 dose escalation trial for non-Hodgkin’s lymphoma (NHL).
Sonny Hsiao, PhD, the Chief Executive Officer, President, and Co-Founder of Acepodia, stated, “Given the observed clinical advantages of CAR-T therapies in autoimmune disorders, we aim to demonstrate that ACE1831 can achieve a more substantial B cell depletion than traditional antibody therapies, which is essential for extending remission periods in autoimmune diseases.” Jerry Liu, MD, the Head of Clinical Development at Acepodia, added, “We have collaborated with leading IgG4-RD specialists globally, who are eager about our pioneering approach. This approval allows us to commence the study and provide participants with a promising new therapeutic option."
The Phase 1b/2a trial of ACE1831 will be conducted in partnership with Pfizer Ignite, a program designed to grant biotech firms access to Pfizer’s extensive resources and expertise to expedite the development of potentially groundbreaking therapies for patients. The trial will be directed by Dr. John Stone, MD, MPH, from Massachusetts General Hospital, who is also the Executive Chairman of The IgG4ward! Foundation and a prominent researcher in IgG4-RD known for his work on the effectiveness of targeted B-cell depletion with rituximab. Dr. Stone expressed, “A significant advancement for patients with IgG4-RD has been the demonstrated efficacy of B cell depletion in managing the disease. I am optimistic not only about the likelihood of achieving deeper B cell depletion with Acepodia’s method but also about its user-friendliness and the potential for reduced side effects compared to other cell-based treatments.”
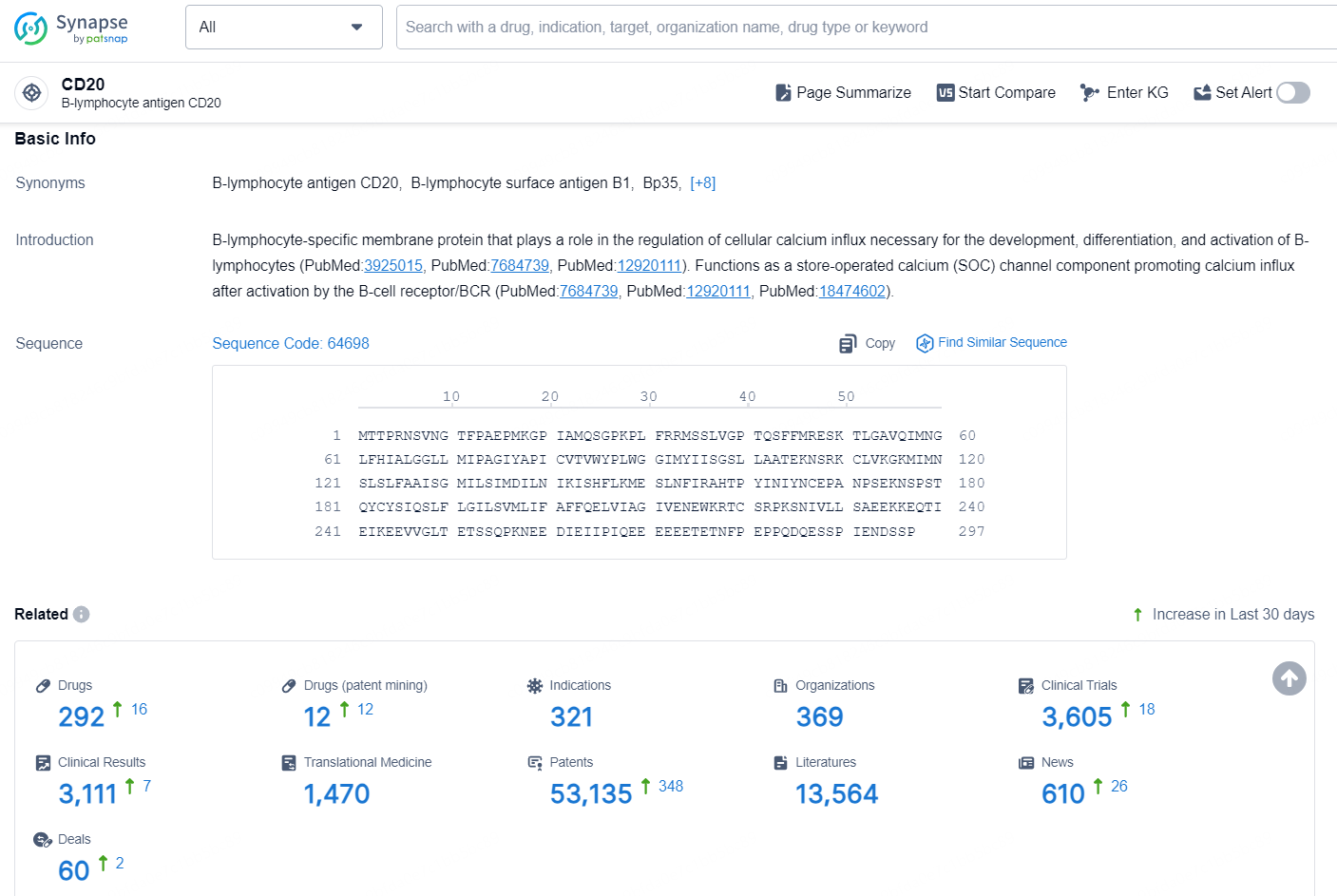
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of November 19, 2024, there are 292 investigational drugs for the CD20 target, including 321 indications, 369 R&D institutions involved, with related clinical trials reaching 3605, and as many as 53135 patents.
ACE-1831 is a gene therapy drug developed by Acepodia Biotech, Inc. The drug falls under the category of gene therapy, specifically targeting γ-δT cells and CD20. It is intended for the treatment of various conditions within the therapeutic areas of Neoplasms, Immune System Diseases, Hemic and Lymphatic Diseases, and Other Diseases. Specifically, it is being investigated for its potential to treat Follicular Lymphoma, Marginal Zone B-Cell Lymphoma, and Mediastinal large B-cell lymphoma.