The Journey of VVD-130037 in Targeting KEAP1 x Nrf2 Pathways for Solid Neoplasms
The drug VVD-130037 is a small molecule drug developed to target the KEAP1 x Nrf2 pathways in the treatment of neoplasms, specifically advanced malignant solid neoplasms. The originator organization of this drug is Vividion Therapeutics, Inc., and it has reached the highest phase of clinical development at Phase 1 globally. Vividion Therapeutics, Inc. focuses on developing innovative small molecule therapeutics for the treatment of cancer and other serious diseases. The drug VVD-130037 is designed to target specific molecular pathways involved in the development and progression of neoplasms, which are abnormal growths of tissue that can be benign or malignant.
Through its mechanism of action on the KEAP1 x Nrf2 pathways, VVD-130037 aims to disrupt the abnormal cellular processes that drive the growth and spread of advanced malignant solid neoplasms. The Phase 1 clinical trial signifies that the drug has been tested in a small number of human subjects to evaluate its safety, dosage, and potential side effects. This early stage of development marks an important milestone towards further understanding the clinical potential of VVD-130037 in the treatment of neoplasms.
Below, we will use the drug VVD-130037 as an example to demonstrate how to quickly obtain information about its chemical structure and patent situation using the Patsnap Chemical.
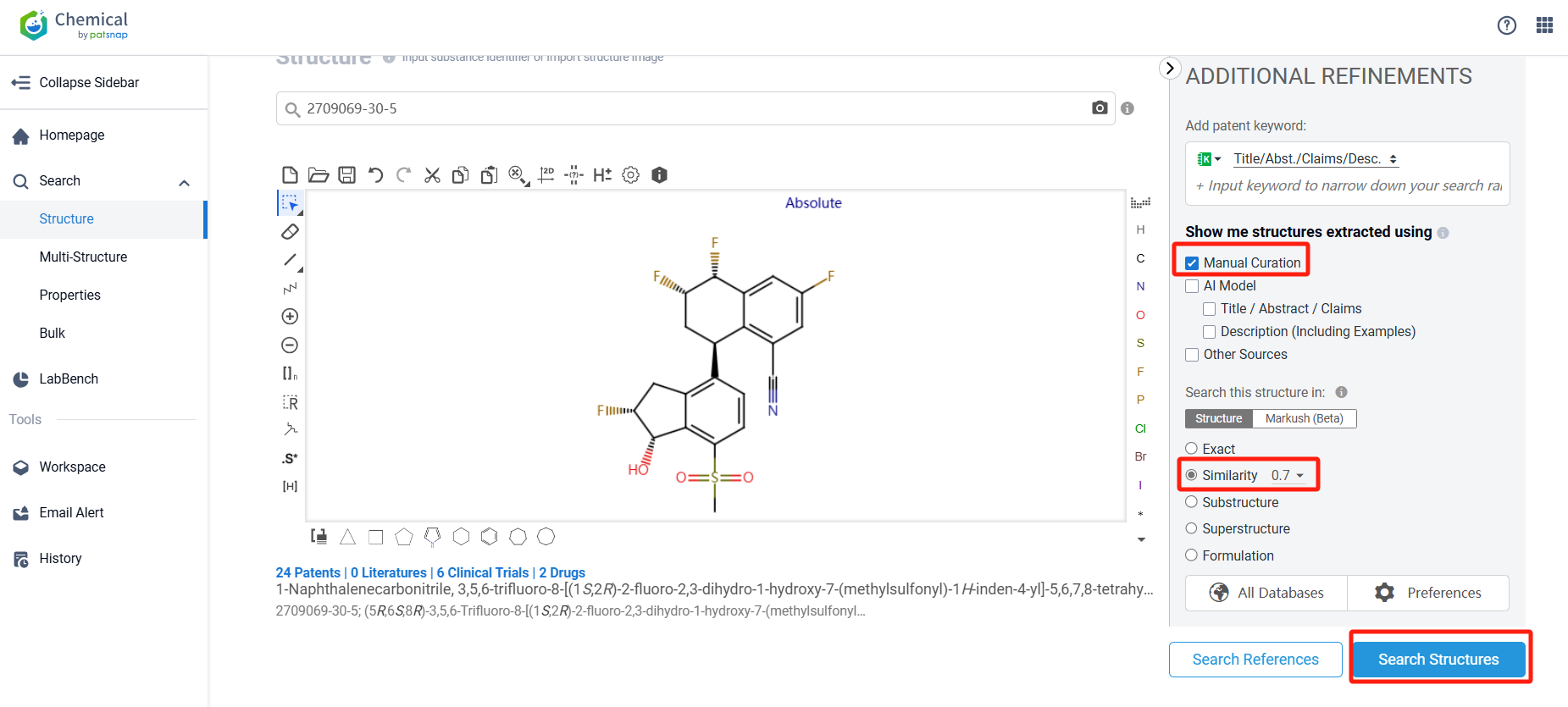
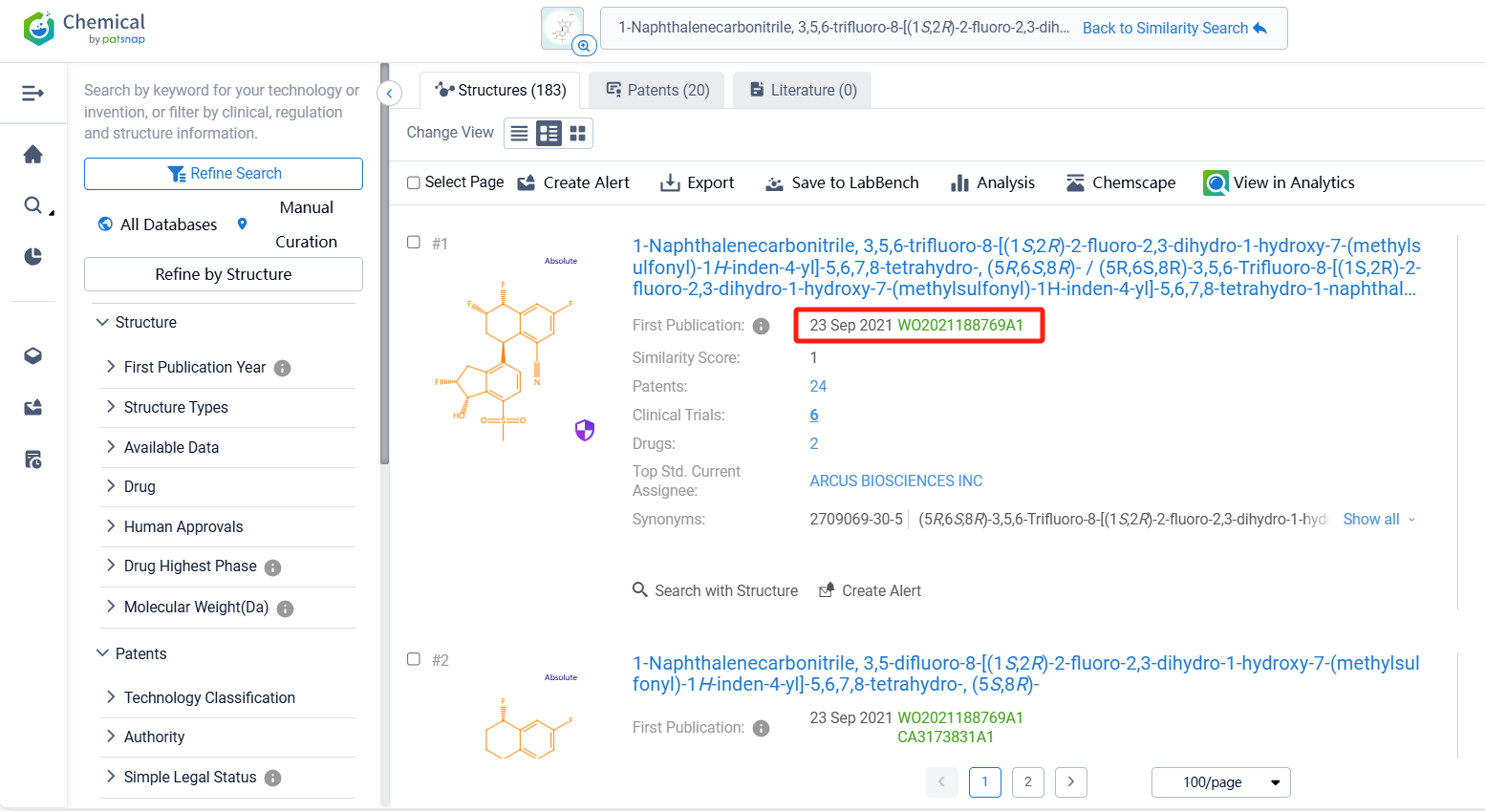
Log in to the Patsnap Chemical. Select the structural search and enter the common identity information of VVD-130037 (such as CAS number, generic substance name, molecular formula, SMILES file, etc.). Here, using a similarity search (setting the Tanimoto coefficient to 0.7), check the box for manual curation, click on search structures, and you can find the innovative drug VVD-130037 as disclosed in the patent application with the publication number WO2021188769A1, first made public on 2021-09-23.
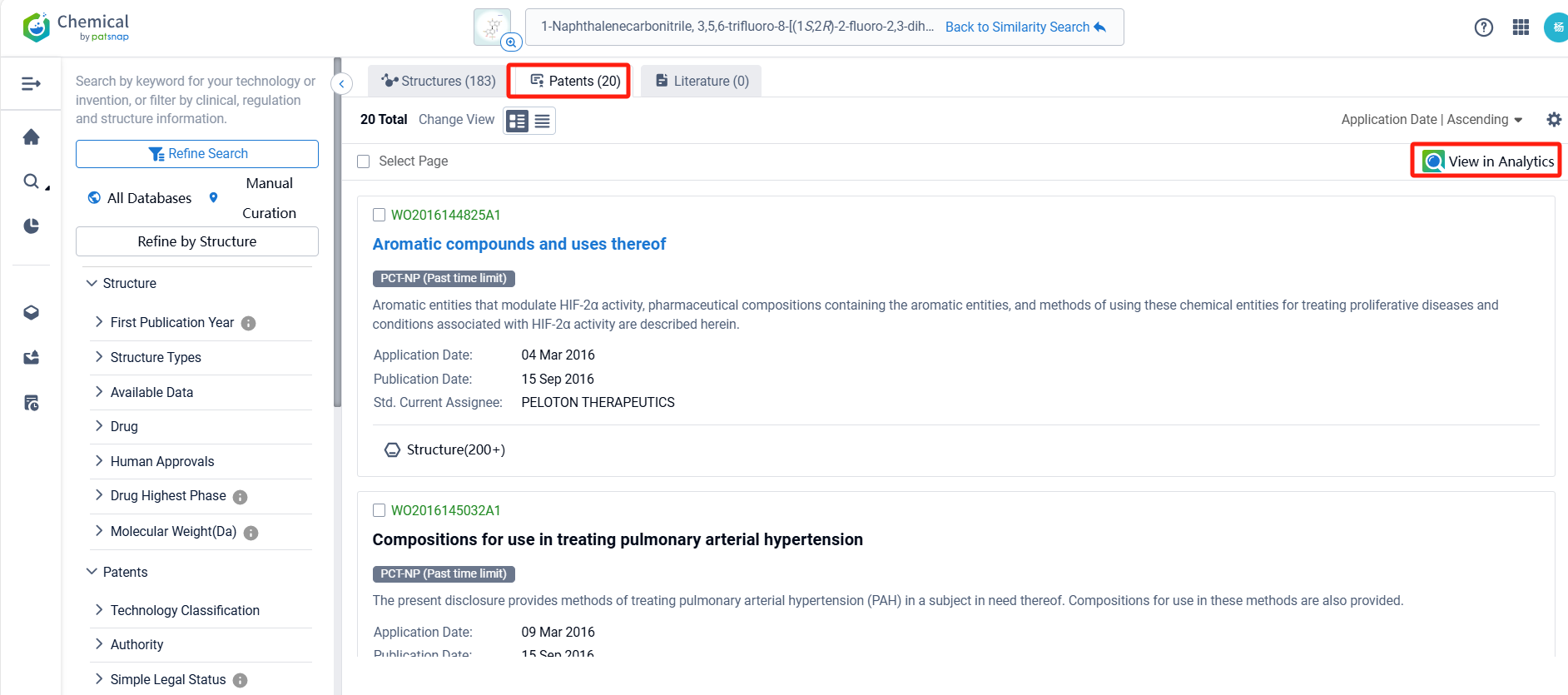
There are 20 patents related to this compound. Clicking the "view in Analytics" will direct you to the Patsnap Patent.
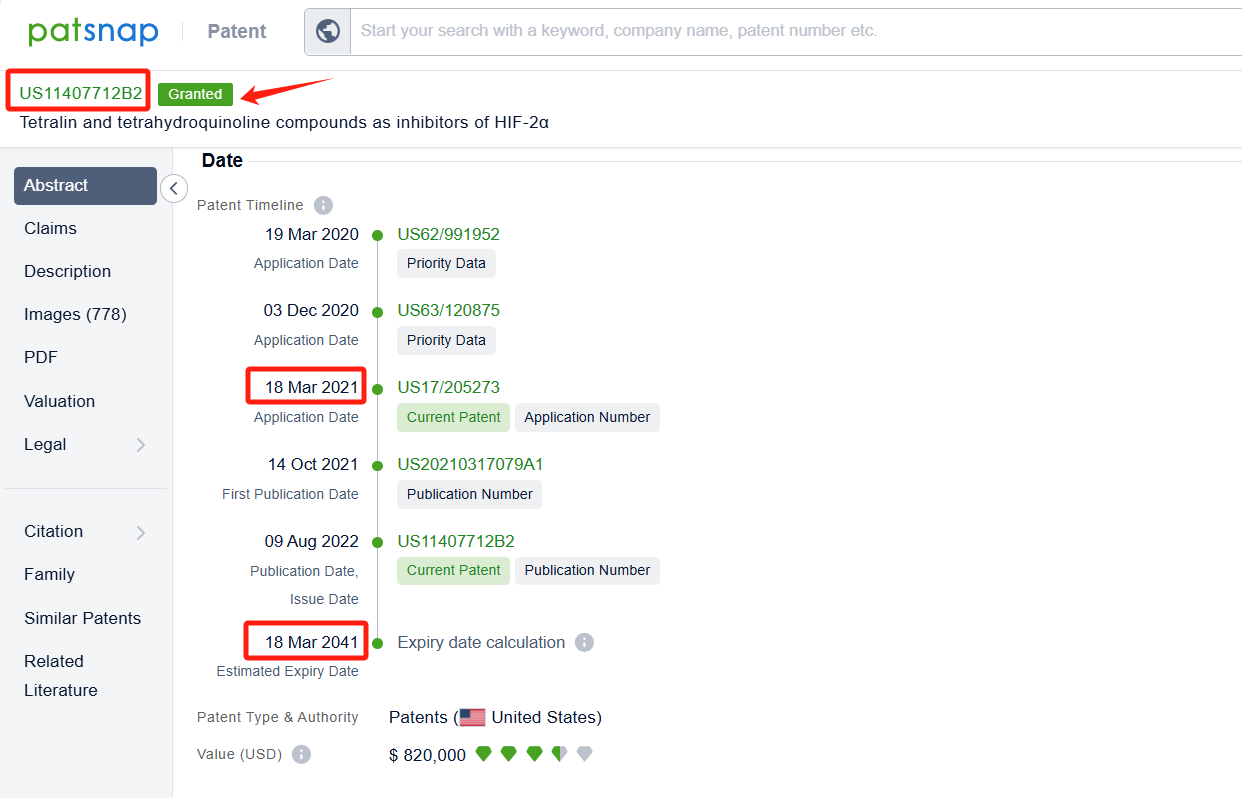
By reviewing the aforementioned patents, we can observe that the core United States patent related to this compound has been granted, with the grant publication number US11407712B2, the grant date being 09 Aug 2022, and the estimated expiration date 18 Mar 2041. The Chinese and Japanese counterparts of the compound have also been granted, with the grant publication numbers CN115298165B and JP7474861B2, respectively.
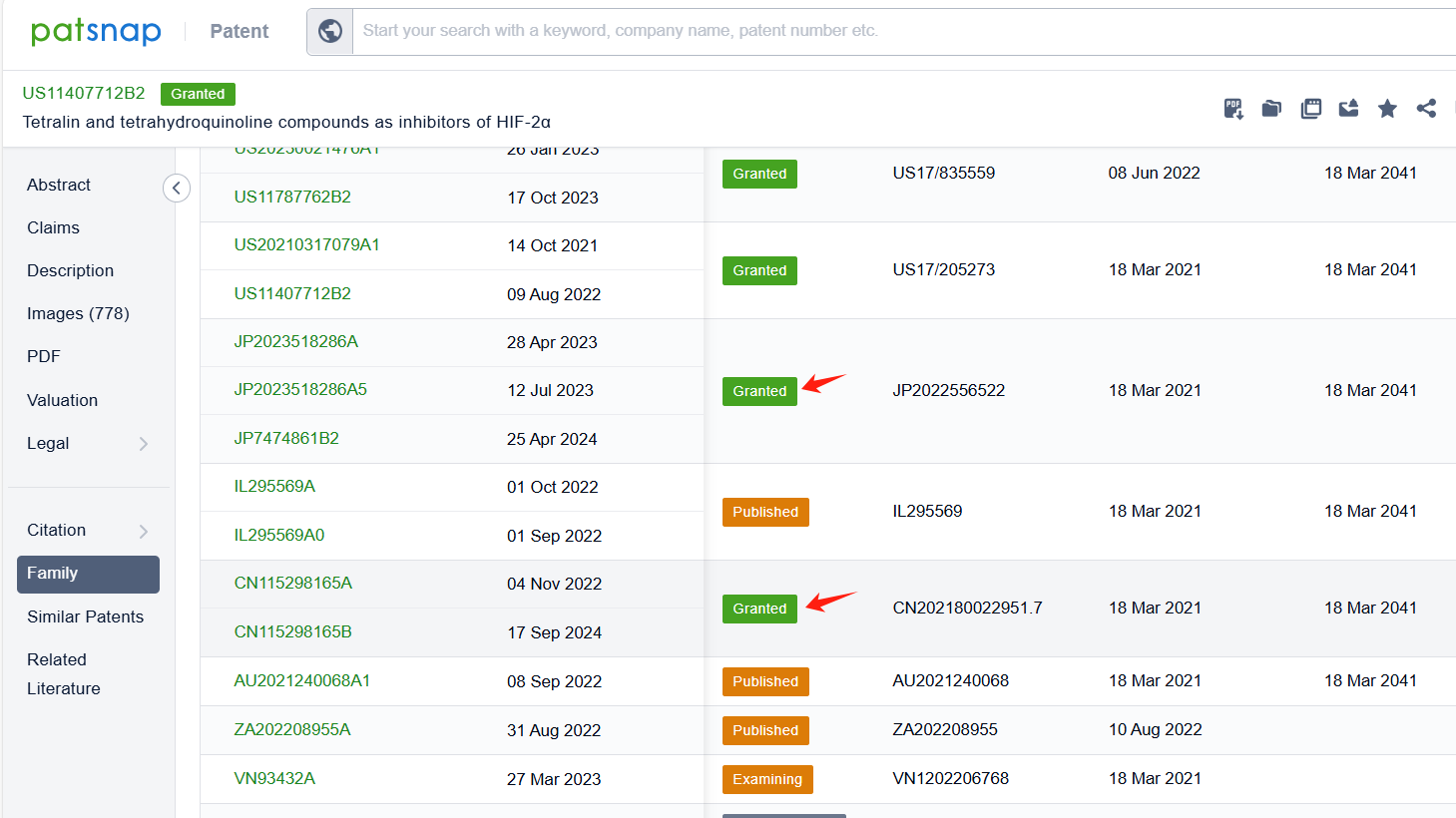
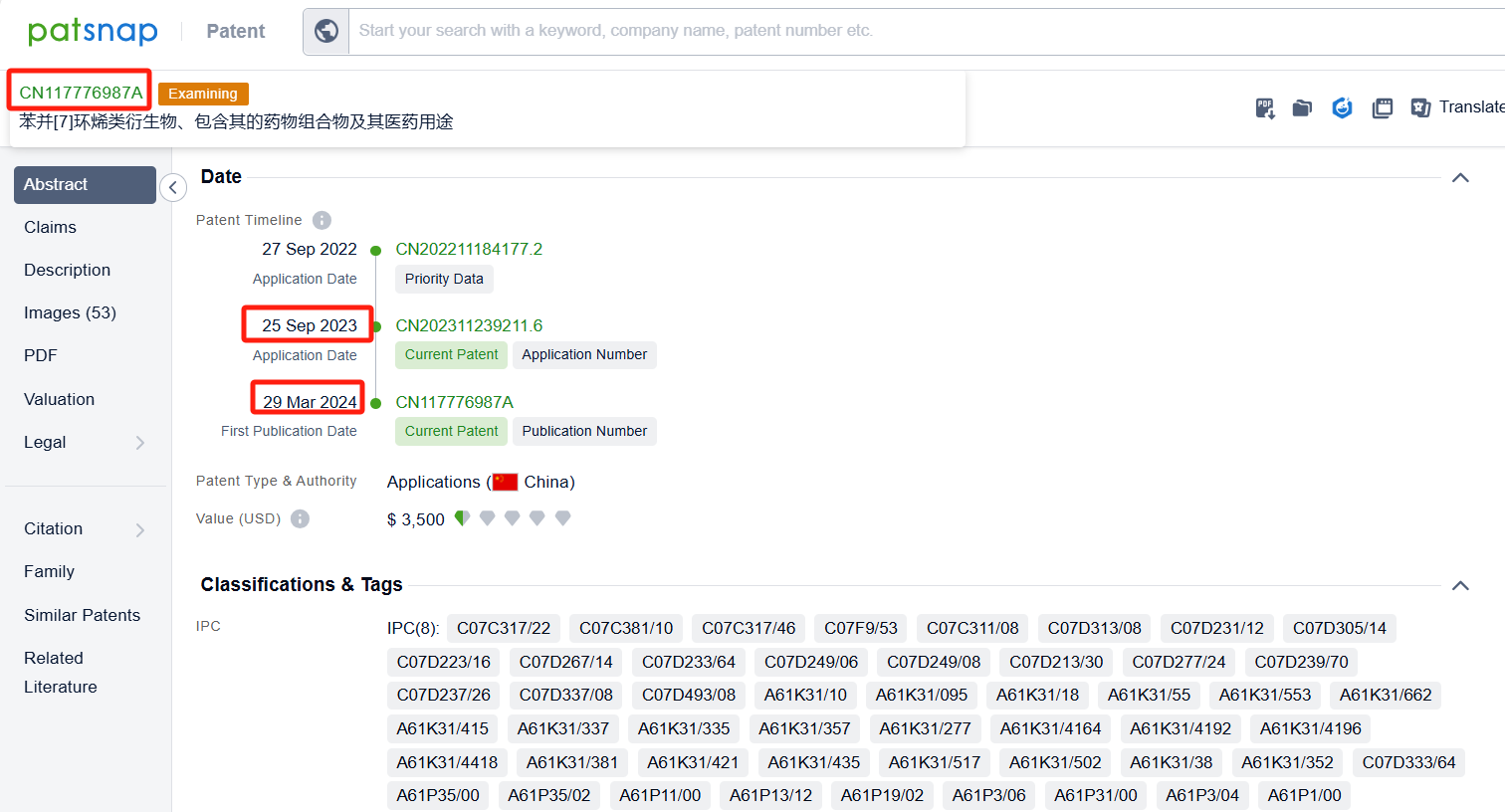
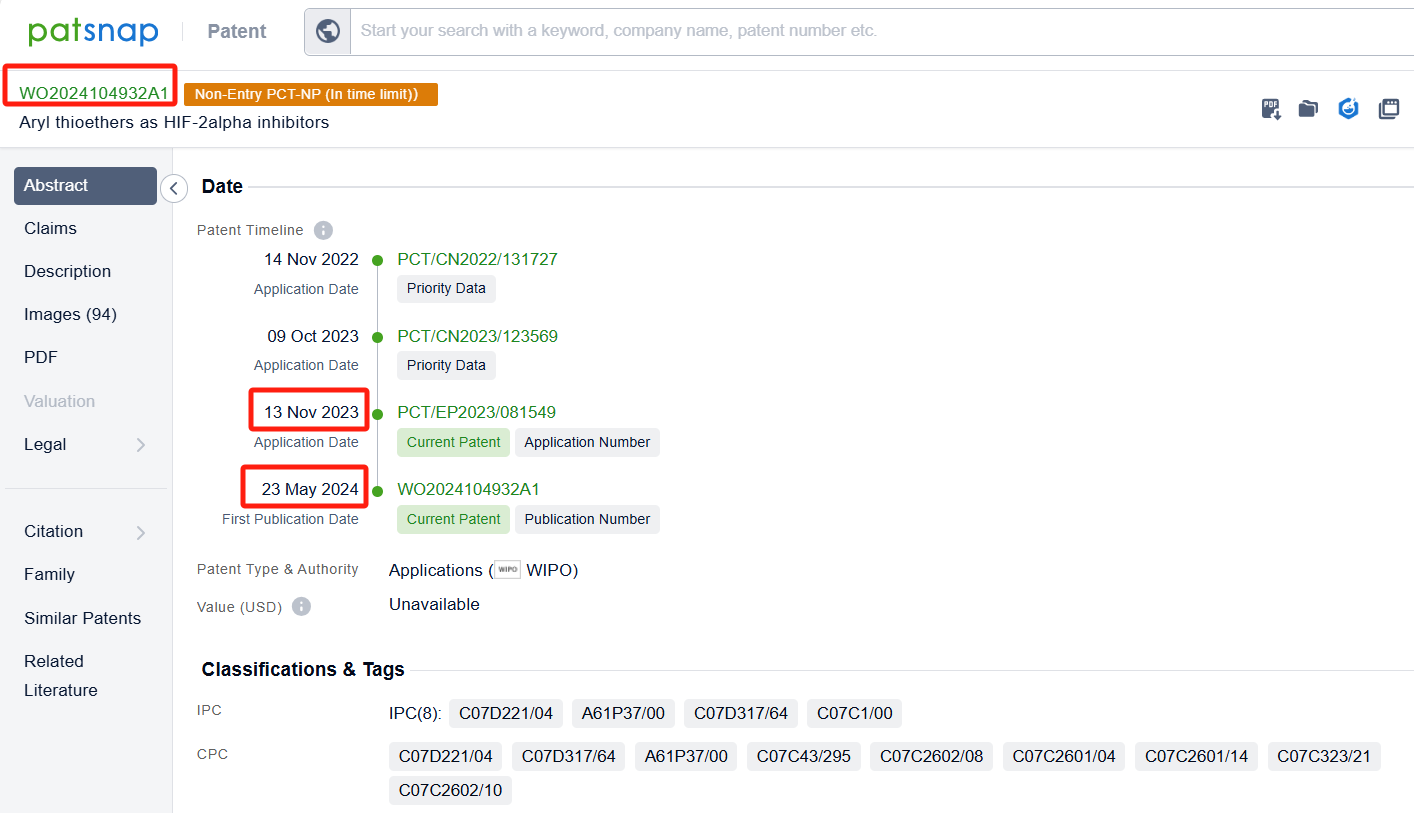
Among the applicants of the patent, one can find other companies' fast follow patents on Arcus Biosciences, Inc.; for example, Suzhou AlphaMa Biotechnology Co., Ltd.'s patent CN117776987A (application date 20230925, publication date 20240329) discloses a benzo[7]cycloalkene derivative, which acts as a type 2α hypoxia-inducible factor (HIF-2α) inhibitor and can be used to treat diseases such as renal cell carcinoma. Additionally, F. Hoffmann-La Roche Ltd.'s patent WO2024104932A1 (application date 20231113, publication date 20240523) provides novel compounds that can inhibit HIF-2α, a protein involved in the pathophysiology of inflammatory bowel disease (IBD). These compounds have superior HIF-2α inhibition activity and good safety margin.
The therapeutic areas targeted by VVD-130037, neoplasms, and specifically advanced malignant solid neoplasms, represent the potential indications for which the drug may be beneficial. The small molecule drug type indicates that VVD-130037 is a low molecular weight compound that has been designed to interact with specific molecular targets within the body, making it a promising candidate for targeted therapy in cancer treatment.
Overall, the development of VVD-130037 represents a significant advancement in the field of biomedicine, particularly in the pursuit of novel treatments for neoplasms. As the drug progresses through the various phases of development, it holds the potential to provide a new therapeutic option for patients with advanced malignant solid neoplasms, addressing a critical unmet medical need in oncology.
AI built to maximize IP and R&D efficiency
Redefine chemical FTO with a range of structure retrieval options at your fingertips, from exact matches to similarity searches, all powered by deep data processing techniques and proprietary AI algorithms to eliminate the risk of omitting key results.