InnoCare Initiates Phase III Trial of TYK2 Inhibitor ICP-332 for Atopic Dermatitis in China
InnoCare Pharma (HKEX: 09969; SSE: 688428), a prominent biopharmaceutical firm specializing in therapies for cancer and autoimmune disorders, has reported that the initial participant has been administered a dose in the Phase III registration trial of its innovative TYK2 (Tyrosine Kinase 2) inhibitor ICP-332 aimed at treating atopic dermatitis (AD) in China.
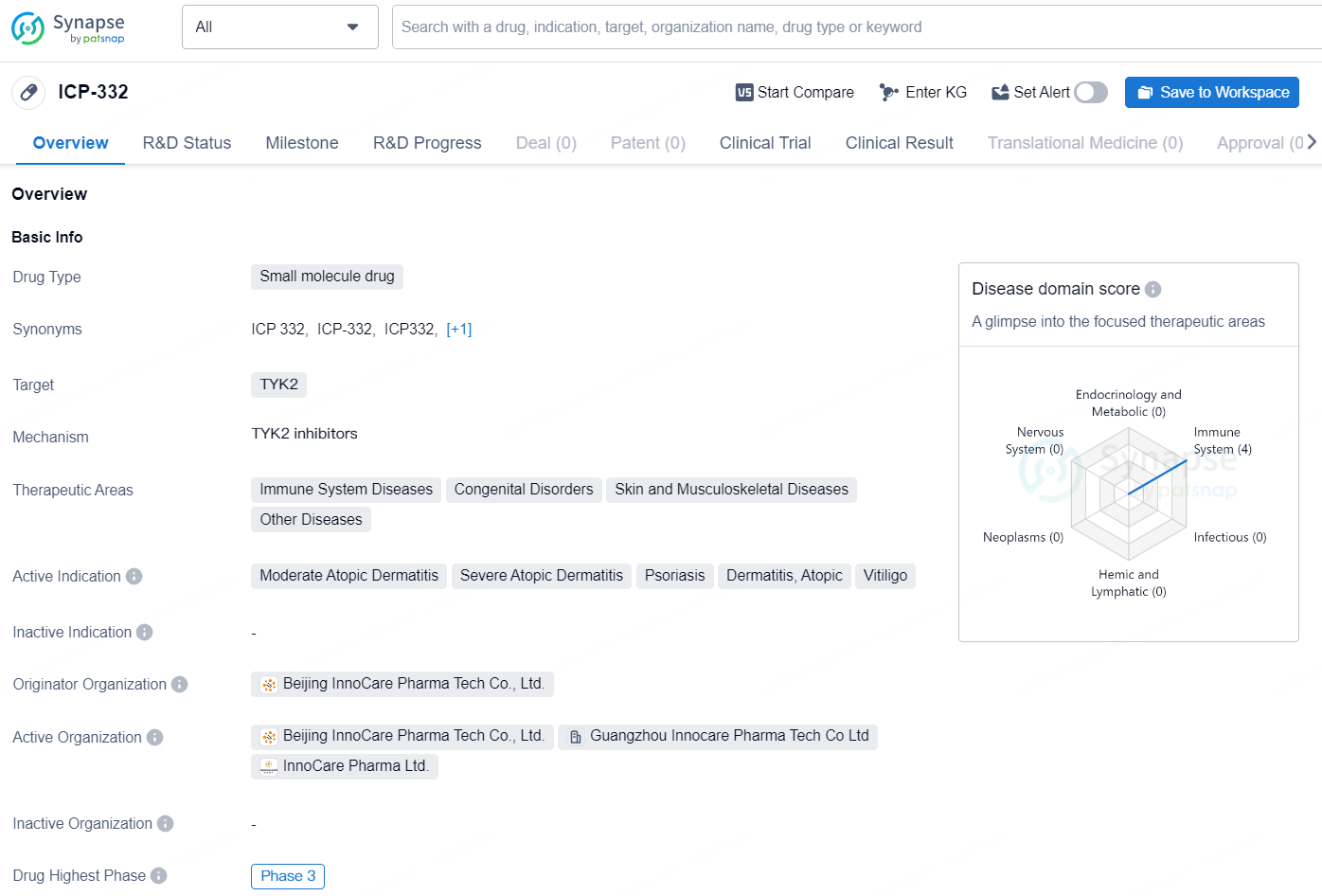
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
ICP-332 is a strong and selective inhibitor of TYK2 currently being developed for the therapeutic management of various autoimmune disorders related to T-cells, such as atopic dermatitis (AD), vitiligo, and inflammatory bowel disease, among others. This compound has significant market potential. As a non-receptor tyrosine kinase, TYK2 belongs to the JAK kinase family, which is a crucial component of the JAK-STAT signaling pathway, playing a vital role in the development of inflammatory conditions.
At present, there are no approved TYK2 inhibitors globally for the treatment of atopic dermatitis. In a Phase II trial conducted in China, ICP-332 met multiple efficacy endpoints for patients suffering from moderate to severe atopic dermatitis, showcasing an excellent safety and efficacy profile. Additionally, ICP-332 exhibited superior efficacy relative to various classes and mechanisms of action (MoAs) of therapies for AD patients, although this was not a direct comparison.
According to the World Health Organization's Global Burden of Disease Study, there are approximately 230 million individuals affected by atopic dermatitis, which represents the highest disease burden among nonfatal skin conditions. The prevalence of atopic dermatitis in China continues to rise annually.
Dr. Jasmine Cui, who is the Co-founder, Chairwoman, and CEO of InnoCare, stated, “Autoimmune diseases can impact nearly every organ in the body and can manifest at any age. At InnoCare, we are committed to pioneering advancements in autoimmune therapy by concentrating on B-cell and T-cell pathways. We have built a strong portfolio of unique therapeutics for autoimmune diseases that hold considerable market prospects globally, including orelabrutinib (a BTK inhibitor), ICP-332 (a TYK2-JH1 inhibitor), ICP-488 (a TYK2-JH2 inhibitor), and a novel small molecule blocker of IL-17. We aim to expedite clinical development and are eager for our innovative therapies to assist patients with autoimmune conditions.”
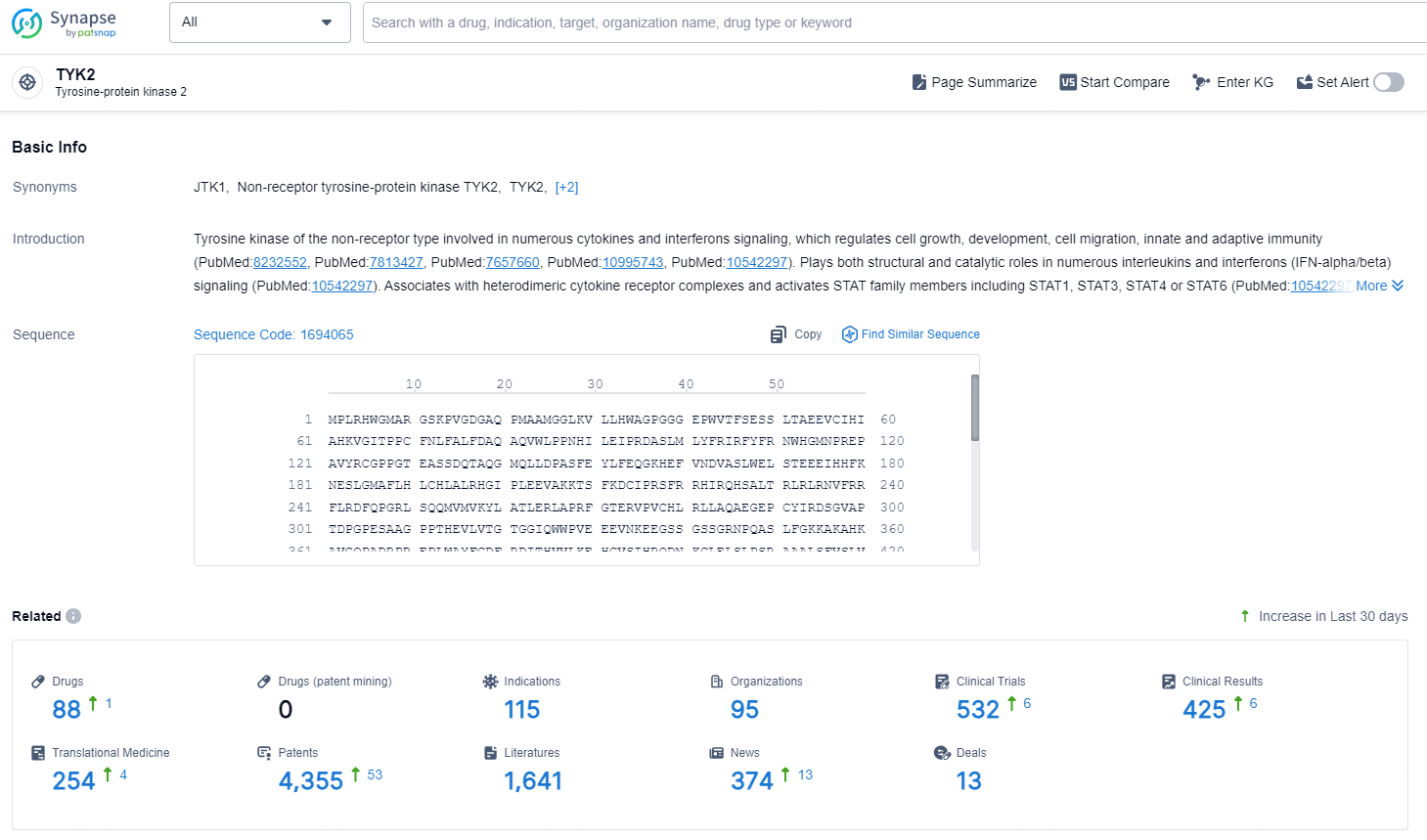
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of November 19, 2024, there are 88 investigational drugs for the TYK2 target, including 115 indications, 95 R&D institutions involved, with related clinical trials reaching 532, and as many as 4355 patents.
ICP-332 is a small molecule drug developed by Beijing InnoCare Pharma Tech Co., Ltd. The drug targets TYK2 and is intended for the treatment of immune system diseases, congenital disorders, skin and musculoskeletal diseases, and other diseases. The active indications for ICP-332 include moderate atopic dermatitis, severe atopic dermatitis, psoriasis, dermatitis, atopic, and vitiligo.