FDA Approves Adicet Bio's IND Amendment for ADI-001 Studies in Idiopathic Inflammatory Myopathy and Stiff Person Syndrome
Adicet Bio, Inc. (Nasdaq: ACET), a biotech firm in the clinical stage that focuses on discovering and developing allogeneic gamma delta T cell therapies for autoimmune disorders and cancer, announced that the U.S. Food and Drug Administration (FDA) has approved an amendment to the Company’s Investigational New Drug (IND) application. This amendment allows for the assessment of ADI-001 in patients with idiopathic inflammatory myopathy (IIM) and stiff person syndrome (SPS) as a part of the ongoing Phase 1 study in autoimmune diseases. The Company is set to begin patient enrollment for IIM and SPS in the first quarter of 2025. This follows the FDA's recent approvals for amendments to the Company’s ADI-001 IND application to explore three more indications in addition to lupus nephritis (LN), which include systemic lupus erythematosus (SLE), systemic sclerosis (SSc), and anti-neutrophil cytoplasmic autoantibody (ANCA)-associated vasculitis (AAV).
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
“The FDA’s approval of our IND amendment to assess ADI-001 in individuals with IIM and SPS enhances our recent progress in the field of autoimmune diseases, broadening our focus to six different autoimmune conditions as we strive to provide our innovative gamma delta T cell therapy candidates to more patients seeking new treatment options,” stated Chen Schor, President and Chief Executive Officer of Adicet Bio. “Following our recent announcement of clinical biomarker findings that showed significant B-cell depletion and targeted trafficking to various tissues and organs, we are confident in ADI-001’s potential to be a leading therapy for autoimmune diseases. We anticipate commencing patient enrollment for IIM and SPS in the first quarter of 2025 as part of our ongoing Phase 1 clinical program.”
The Phase 1 trial for ADI-001 in autoimmune diseases will consist of four distinct arms, with patients suffering from LN and SLE in one arm, SSc patients in another, AAV patients in a third, and IIM and SPS patients placed in the fourth arm. This last cohort will group together various rare autoimmune muscle disorders into a single dose-finding category, which includes SPS and several subtypes of IIM: dermatomyositis, anti-synthetase syndrome, immune-mediated necrotizing myopathy, polymyositis, and overlap myositis. Participants will receive one dose of ADI-001, with the dose-limiting toxicity observed over a 28-day period. Responses and safety will be evaluated on Day 28, as well as during follow-up assessments at 3, 6, 9, 12, 18, and 24 months. The principal aims of the study are to assess the safety and tolerability of ADI-001. Secondary objectives involve evaluating cellular kinetics, pharmacodynamics, variations in autoantibody levels, and relevant disease activity scores within each condition.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
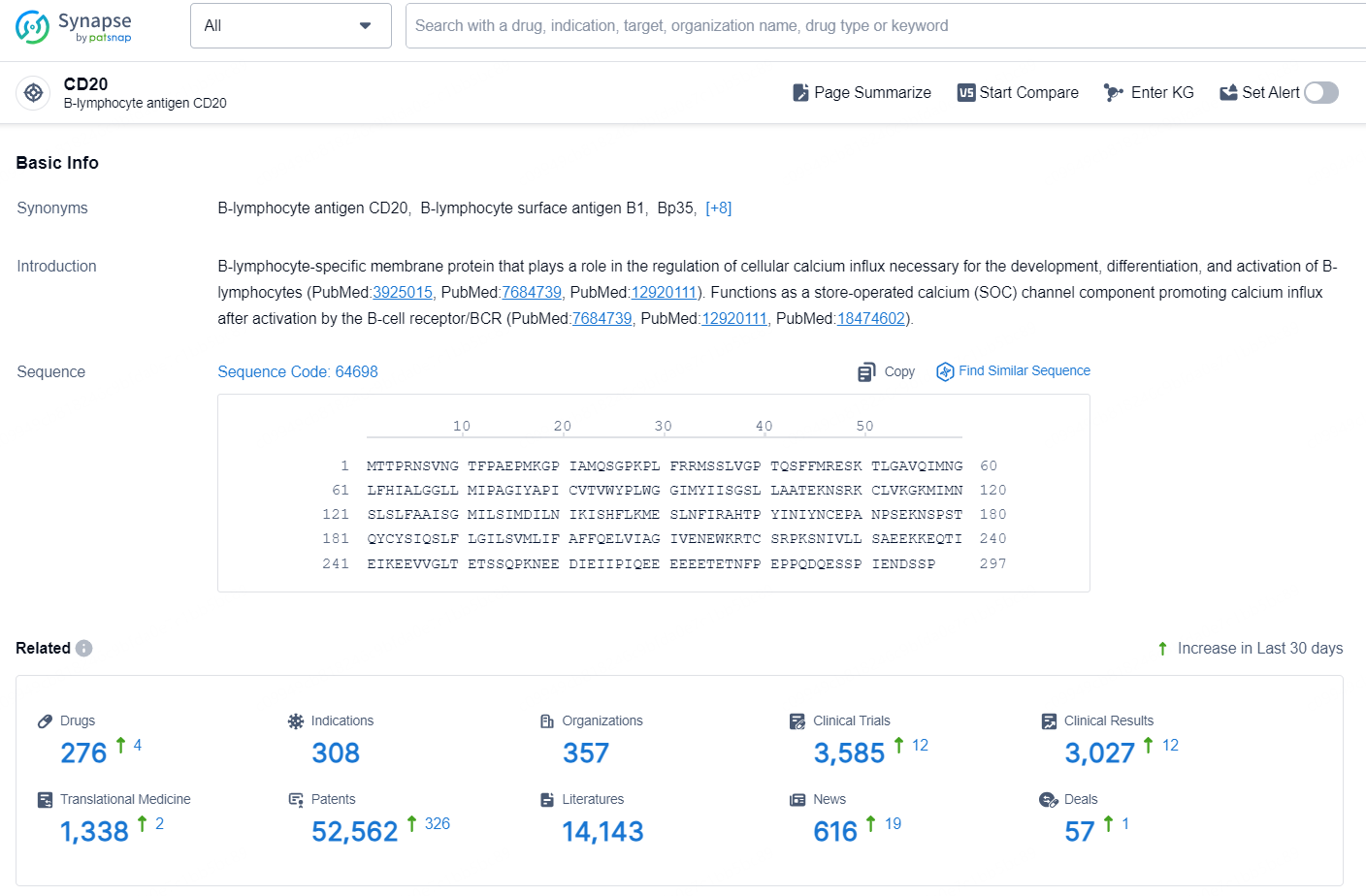
According to the data provided by the Synapse Database, As of October 18, 2024, there are 276 investigational drug for the CD20 target, including 308 indications, 357 R&D institutions involved, with related clinical trials reaching 3585, and as many as 52562 patents.
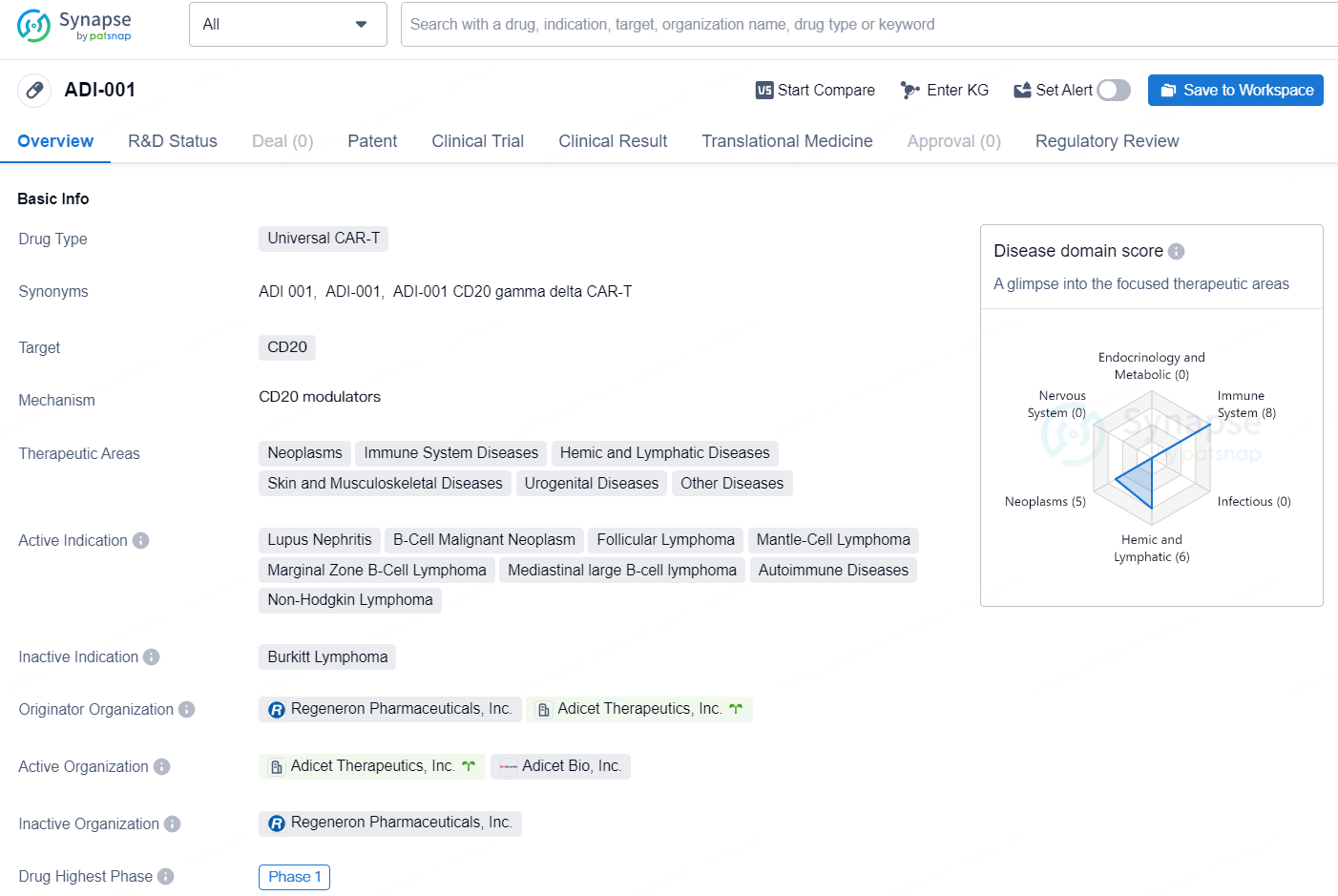
ADI-001 is a Universal CAR-T therapy developed to target CD20, with a focus on therapeutic areas such as neoplasms, immune system diseases, hemic and lymphatic diseases, skin and musculoskeletal diseases, urogenital diseases, and other diseases. The active indications for ADI-001 include lupus nephritis, B-cell malignant neoplasm, follicular lymphoma, mantle-cell lymphoma, marginal zone B-cell lymphoma, mediastinal large B-cell lymphoma, autoimmune diseases, and non-Hodgkin lymphoma.