Tenaya Therapeutics Updates on TN-201 Gene Therapy for MYBPC3-related HCM
Tenaya Therapeutics, Inc. (NASDAQ: TNYA), a biotech company in the clinical trial phase, aims to discover, develop, and provide potentially curative treatments for the root causes of heart disease. The company has released updates concerning its ongoing Phase 1b/2 MyPEAK-1 clinical study of TN-201. This investigational therapy targets MYBPC3-associated hypertrophic cardiomyopathy (HCM), a condition resulting from inadequate amounts of myosin-binding protein C (MyBP-C). The TN-201 gene replacement therapy is intended to enhance MyBP-C protein levels, potentially slowing or reversing disease progression by introducing a functional MYBPC3 gene into cardiac muscle cells.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
“The MyPEAK-1 trial evaluating TN-201 primarily aims to determine the safety profile of this gene therapy. We are pleased to announce that TN-201 exhibits a favorable tolerability profile at the 3E13 vg/kg dose, with no unforeseen adverse effects,” stated Whit Tingley, M.D., Ph.D., Chief Medical Officer at Tenaya. “The Data and Safety Monitoring Board’s (DSMB) recommendation to move forward with dose escalation and their support for an expanded eligibility criterion are optimistic early signs regarding the safety of TN-201. These developments allow us to investigate the applicability of TN-201 in various patient populations. We have made several protocol modifications, such as including a baseline biopsy, broadening eligibility to obstructive HCM patients, and enlarging the overall scope of the MyPEAK-1 study.”
Tenaya has successfully completed dosing for the first three participants (Cohort 1) at the 3E13 vg/kg dose in the MyPEAK-1 trial, without any unexpected events or associated toxicities with the study medication. The safety data from Cohort 1 has been assessed by an independent DSMB, revealing a safety and tolerability profile in line with other AAV gene therapies at this dosage. Following this, the DSMB has advised Tenaya to escalate the dose to 6E13 vg/kg for Cohort 2, as stipulated in the protocol. Recruitment for Cohort 2 has commenced.
Tenaya is implementing a number of modifications to the MyPEAK-1 protocol to facilitate future development, which include:
- Introducing a baseline biopsy, increasing the total number of cardiac biopsies from two to three, and allowing more flexibility in the timing of post-dose biopsies to assess TN-201 expression over time.
- Expanding eligibility criteria to allow participants without implantable cardioverter defibrillator (ICD) devices and permitting adults with either obstructive or nonobstructive HCM to participate.
- Increasing the potential number of participants in the dose expansion phase of the clinical trial from nine to twenty-four adults.
Tenaya intends to share initial findings from Cohort 1 in December of this year, focusing on the safety and tolerability of TN-201, analyses of cardiac biopsy results, and changes in cardiac biomarkers from baseline. The MyPEAK-1 clinical trial is also gathering data to evaluate TN-201’s impact on imaging biomarkers, heart functionality, exercise capacity, functional status, and overall patient quality of life.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
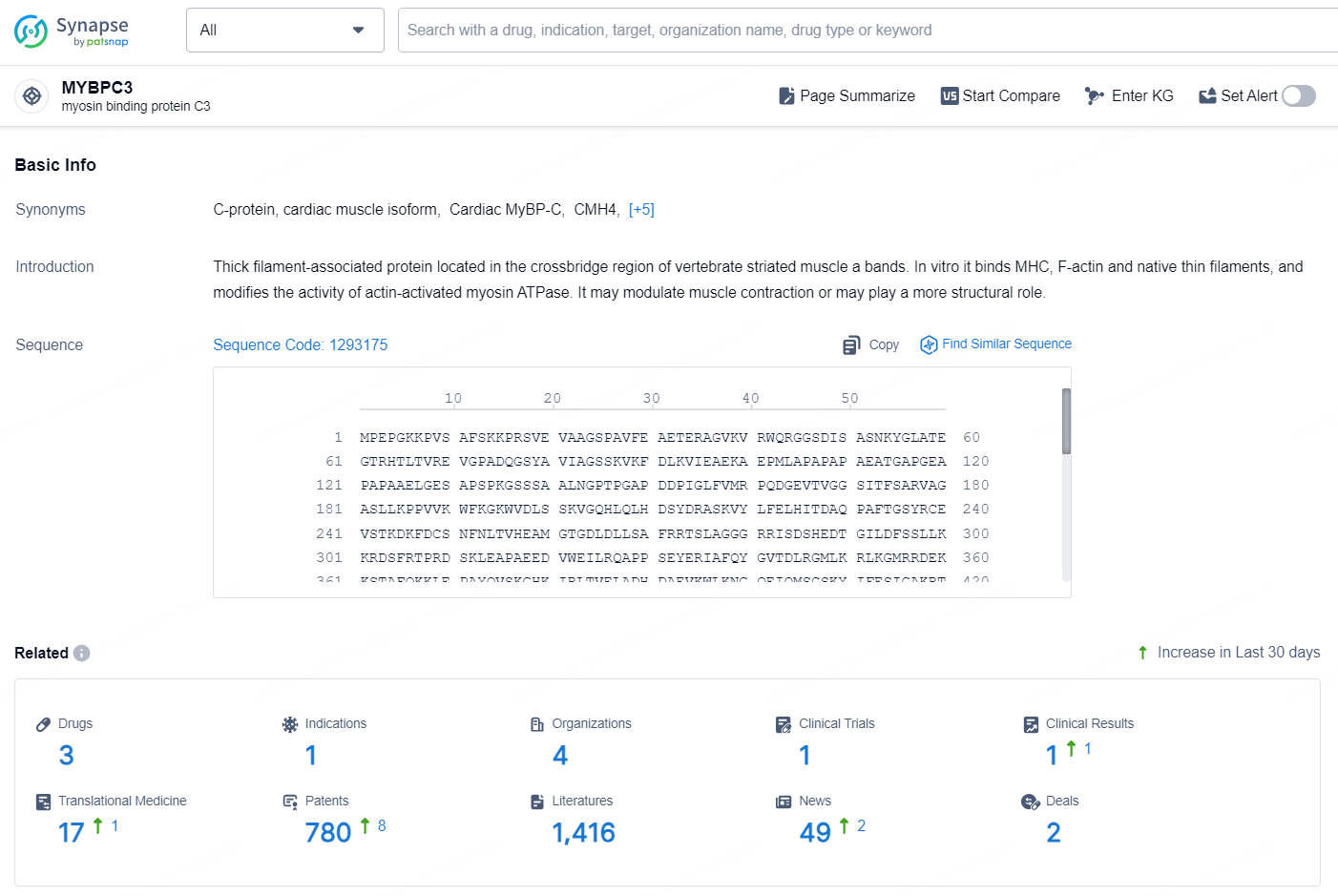
According to the data provided by the Synapse Database, As of October 23, 2024, there are 3 investigational drugs for the MYBPC3 target, including 1 indication, 4 R&D institutions involved, with related clinical trial reaching 1, and as many as 780 patents.
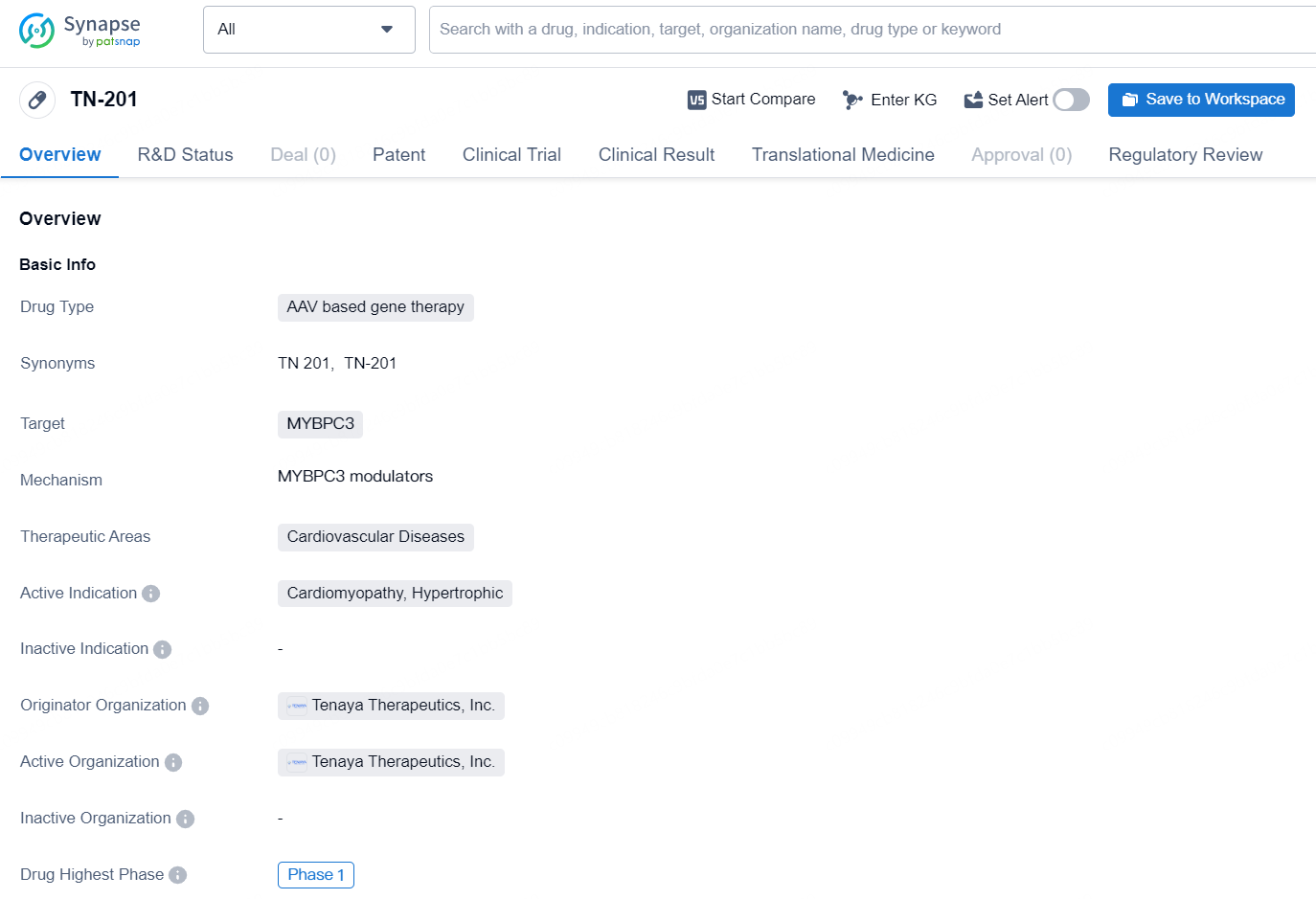
The drug TN-201 is an AAV based gene therapy developed by Tenaya Therapeutics, Inc. The drug targets MYBPC3 and is intended for the treatment of Cardiovascular Diseases, specifically for Cardiomyopathy, Hypertrophic. As of the latest information available, the drug is in Phase 1 of development, indicating that it is still in the early stages of clinical trials.