FDA Approves Galderma's Nemluvio® for Moderate to Severe Atopic Dermatitis
Galderma (SWX:GALD) has announced that the Food and Drug Administration (FDA) of the United States has granted approval for Nemluvio® (nemolizumab) aimed at treating individuals aged 12 and older who suffer from moderate-to-severe atopic dermatitis. This treatment is intended to be used alongside topical corticosteroids (TCS) and/or calcineurin inhibitors (TCI) when the condition is not sufficiently managed by existing topical prescription drugs. This approval comes after the FDA's earlier authorization of Nemluvio for subcutaneous administration as a treatment for adults with prurigo nodularis in August 2024.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Atopic dermatitis impacts over 230 million individuals globally, affecting around 7% of the U.S. population. Among various symptoms, itchiness is frequently cited as one of the most troublesome by patients, with 87% of those suffering from atopic dermatitis expressing a desire for relief from itching. Consequently, both patients and healthcare providers prioritize rapid itch alleviation. Furthermore, atopic dermatitis is a highly diverse condition that may be linked to various comorbidities, including mental health issues and other autoimmune or immune-mediated disorders. As such, there is an ongoing demand for innovative and effective treatment solutions. Although existing therapies can alleviate some symptoms of atopic dermatitis, many patients do not achieve optimal results and continue to struggle with itch and skin clarity.
Nemluvio is the first monoclonal antibody approved specifically to target the IL-31 receptor alpha, effectively blocking IL-31 signaling. IL-31 is a cytokine involved in neuroimmune processes that promotes itching and contributes to inflammation and skin dysregulation in atopic dermatitis.
The approval of Nemluvio is supported by favorable findings from the phase III ARCADIA clinical trial program, which assessed its safety and efficacy when combined with background topical corticosteroids (TCS), with or without topical calcineurin inhibitors (TCI), versus a placebo group receiving TCS, with or without TCI, in a cohort of 1,728 patients aged 12 and older who have moderate to severe atopic dermatitis.
Data revealed that patients treated with Nemluvio, given through subcutaneous injection every four weeks alongside TCS, with or without TCI, exhibited significant improvements in skin clearance in both of the study's primary endpoints. Specifically, these endpoints included achieving complete (0) or nearly complete (1) clearance of skin lesions as assessed by the investigator’s global assessment (IGA) score, and obtaining a 75% reduction in the Eczema Area and Severity Index (EASI) compared to placebo plus TCS, with or without TCI, after 16 weeks of treatment.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
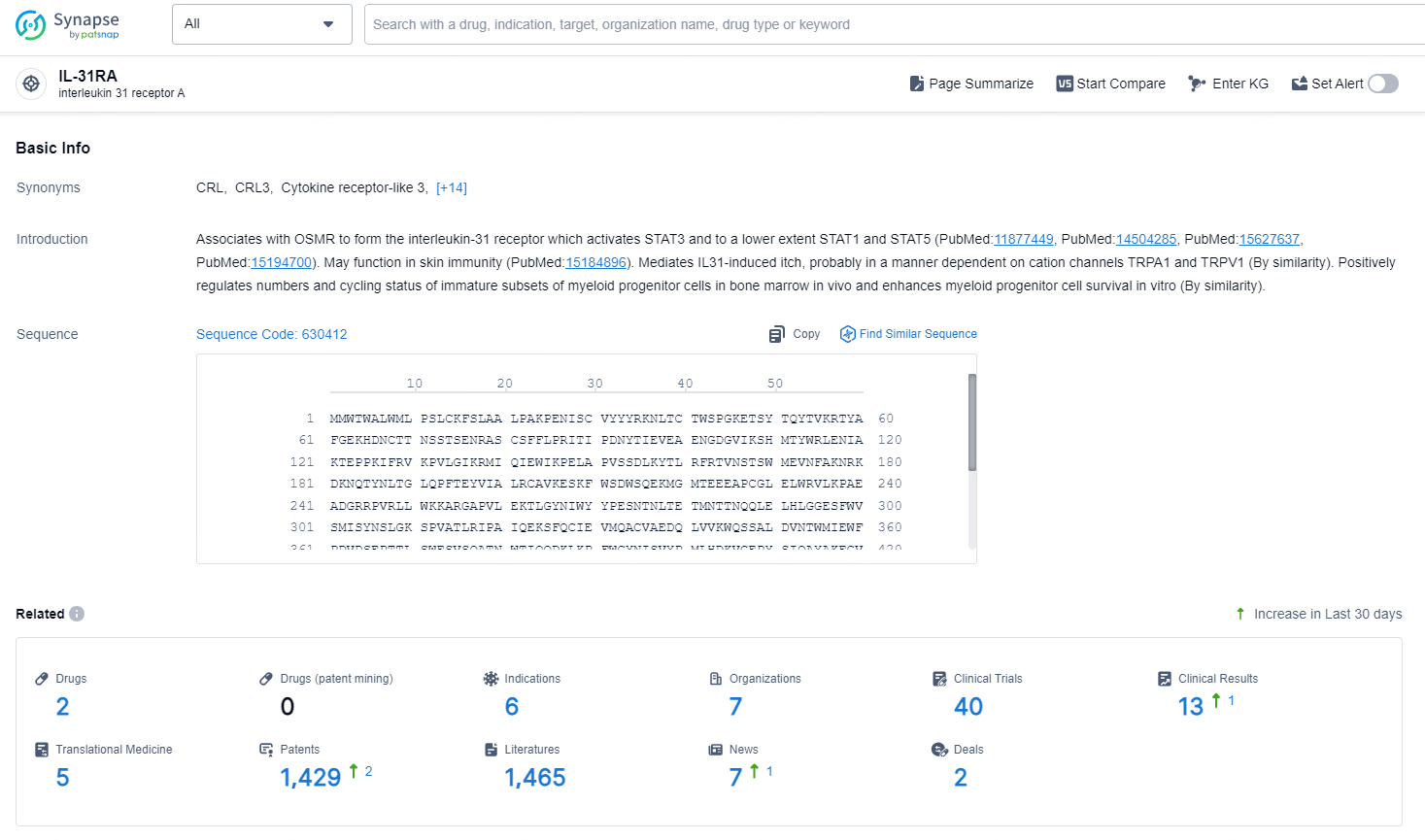
According to the data provided by the Synapse Database, As of December 20, 2024, there are 2 investigational drugs for the IL-31RA target, including 6 indications, 7 R&D institutions involved, with related clinical trials reaching 40 and as many as 1429 patents.
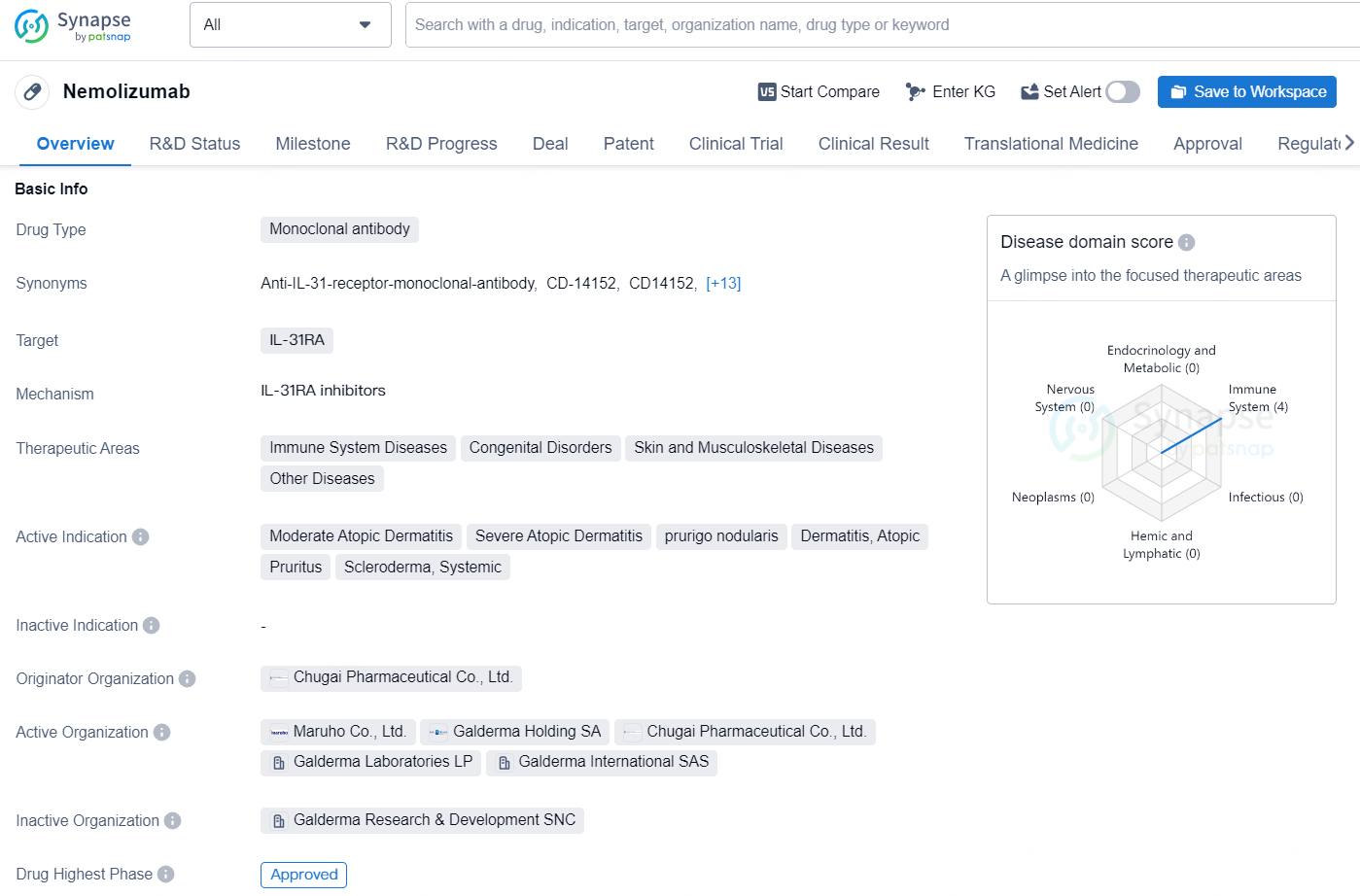
Nemolizumab is a monoclonal antibody drug that targets IL-31RA, with therapeutic areas including immune system diseases, congenital disorders, skin and musculoskeletal diseases, as well as other diseases. The drug is indicated for the treatment of moderate atopic dermatitis, severe atopic dermatitis, prurigo nodularis, dermatitis, atopic, pruritus, and systemic scleroderma. Nemolizumab was developed by Chugai Pharmaceutical Co., Ltd. and received its first global approval in Japan in March 2022.