FDA Approves NK CellTech's Clinical Trials for Therapy NK010
On the 17th of January in the year 2024, the prominent biotechnology enterprise NK CellTech Co., Ltd., which specializes in creating NK cell treatments, proudly disclosed that the United States Food and Drug Administration has authorized the commencement of human testing for their innovative product NK010. This groundbreaking therapy consists of non-genetically altered natural killer cells, marking the inaugural occasion that the FDA has endorsed a non-genetically engineered NK cell therapy derived from allogeneic peripheral blood for a clinical trial that originates from China.
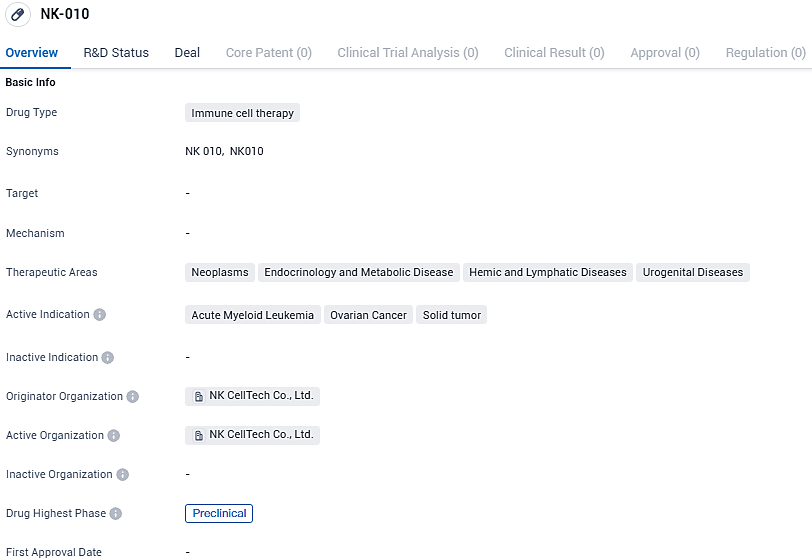
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
NK010 demonstrates numerous anti-cancer properties such as a broad range of efficacy, prominent expression of NK cell activation markers, and exceptional purity. These features equip it to address diverse tumor forms. Additionally, NK010 possesses the capacity to extend its therapeutic use to non-cancerous ailments and holds the potential to become the foundational cellular component for the company's upcoming line of engineered NK cell therapeutics.
For this Phase I clinical exploration, ovarian cancer was the initial target. Preclinical findings revealed impressive effects, with NK010 significantly curtailing the progression of ovarian tumor in animal subjects, and showing efficacy against liver cancer, various solid malignancies, and acute myeloid leukemia as well.
Professor Zhigang Tian, originator of NK CellTech, and a distinguished member of the Chinese Academy of Engineering and the Academia Europaea, expressed his excitement over obtaining FDA authorization to proceed with clinical assessments of NK010. "Our preliminary investigations have exceedingly affirmed the therapeutic promise and safety profile of NK010 in treating solid tumors. While the results thus far bolster our optimism, further investigation is necessary, and our group is dedicated to pioneering new cell-based cancer therapies and to address clinical voids in the future," said Professor Tian.
NK-010 is categorized within the domain of biomedical research, currently advancing through preclinical trials on a global scale, including in China. The medication is being crafted to impact a range of health issues such as cancerous disorders, endocrine and metabolic related conditions, blood and lymph diseases, and disorders affecting the urinary and reproductive systems.
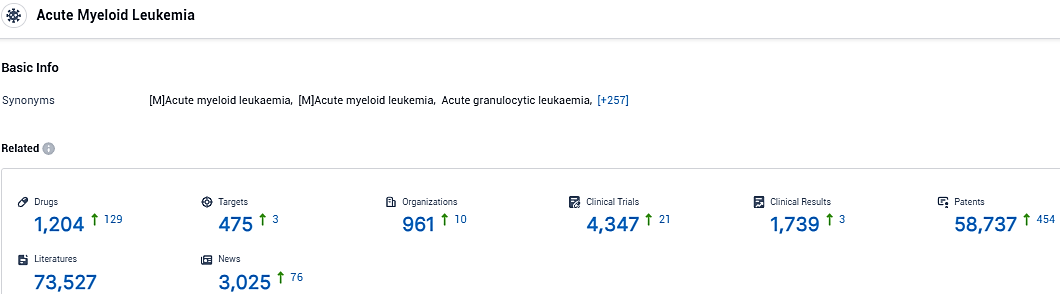
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, targets, organizations, clinical trials, clinical results, and drug patents related to this indication.
According to the data provided by the Synapse Database, As of January 26, 2024, there are 1204 investigational drugs for the Acute Myeloid Leukemia, including 475 targets, 961 R&D institutions involved, with related clinical trials reaching 4347, and as many as 58737 patents.
NK-010 has the potential to be a significant advancement in the field of biomedicine, particularly in the treatment of neoplasms, endocrinology and metabolic disease, hemic and lymphatic diseases, and urogenital diseases. However, further research and clinical trials are needed to determine its safety and efficacy.