ProMIS Neurosciences Reaches Significant Progress in Crafting a Treatment-Based Alpha-Synuclein Immunization
ProMIS Neurosciences Inc., a biotech firm dedicated to creating and advancing treatments that specifically target harmful misshaped proteins implicated in neurodegenerative conditions, has declared the identification of a premier vaccine contender, PMN400. This candidate is designed to combat a range of synucleinopathies, such as Multiple System Atrophy, Parkinson’s disease, and Lewy Body Dementia.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
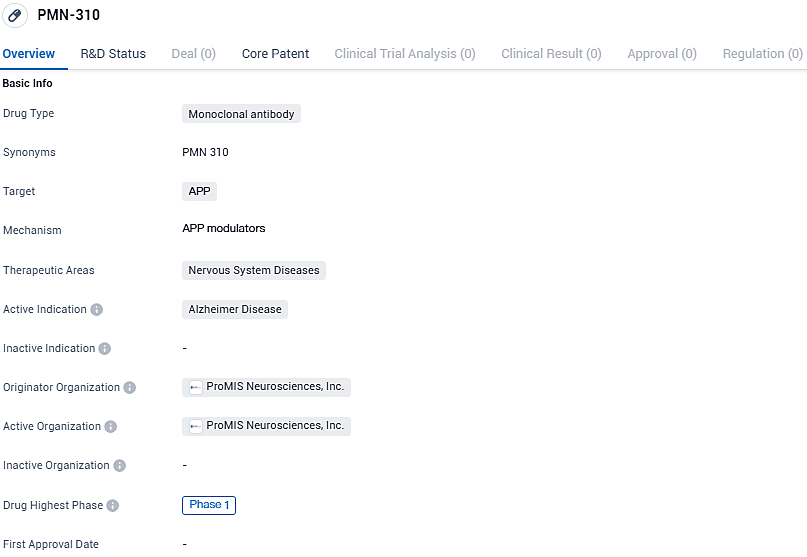
"We are thrilled by the possibilities our innovative technology holds for treating neurodegenerative disorders. At present, we're keenly concentrating on advancing the clinical progression of our top antibody prospect, PMN310, which is moving through a Phase 1a clinical trial aimed at addressing Alzheimer’s disease. Additionally, our platform's capabilities for crafting new, precise antibody treatments and vaccines bring us optimism, offering new approaches to tackle a range of these incapacitating conditions," declared Neil Warma, ProMIS Neurosciences’ CEO.
Neil Warma added, “It's crucial to acknowledge the grant which backs this endeavor. It has facilitated the selection of this auspicious pharmaceutical agent and potentially expands our platform's reach in addressing multiple neurodegenerative conditions through specific antibody medicines and vaccines, aspiring to deliver groundbreaking remedies to those in dire need.”
The innovative endeavors that have propelled this developmental phase were funded by a significant research grant of $1.16 million awarded by the Weston Family Foundation to the University of British Columbia. This grant endorsed the investigative efforts spearheaded by Neil Cashman, M.D., ProMIS' Chief Scientific Officer and Professor Emeritus at the University of British Columbia.
ProMIS leveraged a distinctive computational method to pinpoint unique conformational epitopes associated with the toxic alpha-synuclein implicated in synucleinopathies. Experiments administering a variety of these epitopes in mouse vaccine trials resulted in the selection of a premier vaccine candidate. This candidate is now undergoing tests in mouse models that emulate cognitive and motor impairments observed in human diseases.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
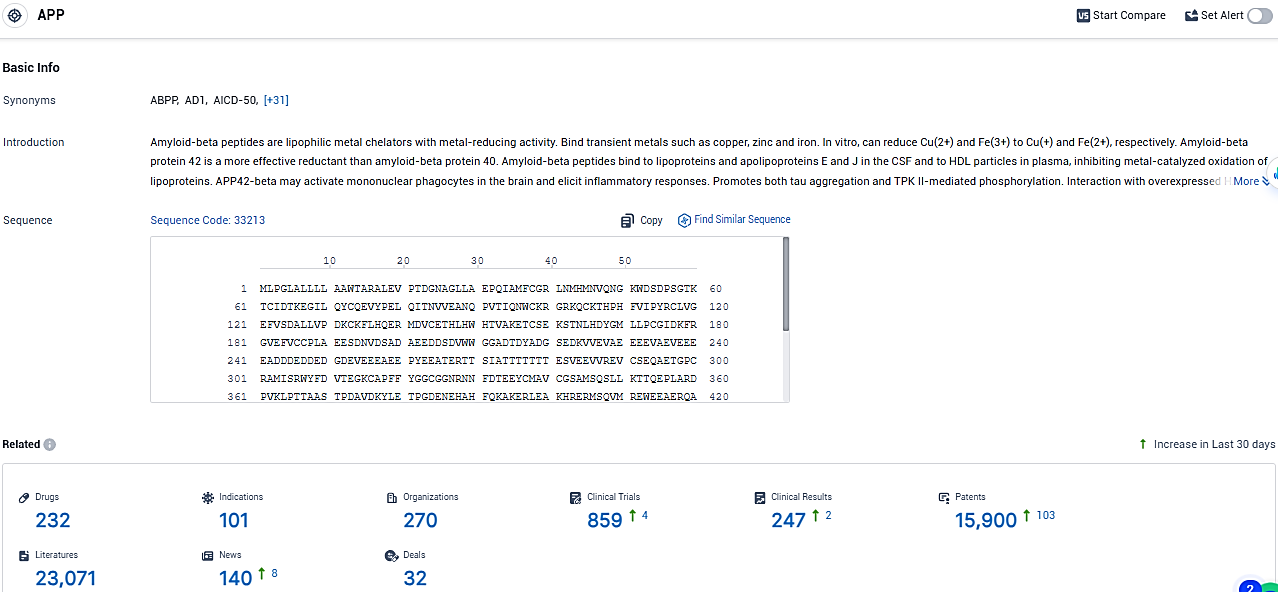
According to the data provided by the Synapse Database, As of January 26, 2024, there are 232 investigational drugs for the APP target, including 101 indications, 270 R&D institutions involved, with related clinical trials reaching 859, and as many as 15900 patents.
PMN-310 targets APP and is being developed for the treatment of Alzheimer's disease, a type of nervous system disease. As of now, it is in Phase 1 of clinical development, and further studies will be needed to determine its efficacy and safety.