Sandoz declares deal to buy CIMERLI® operations from Coherus, reinforcing its foothold in the US sector
Sandoz, a worldwide pioneer in the production of generic and biosimilar pharmaceuticals, has finalized a deal to purchase Coherus BioSciences, Inc. 's biosimilar version of ranibizumab, known as CIMERLI®* (ranibizumab-eqrn), in the United States. The agreement involves an immediate cash transaction of USD 170 million. The purchase encompasses the biologics license submission, existing product stock, a team specialized in ophthalmology marketing and on-the-ground reimbursement expertise, and rights to specialized commercial management software.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
 Keren Haruvi, the head of Sandoz operations in North America, expressed satisfaction with the introduction of another significant addition to the growing collection of biosimilar products under Sandoz. This expansion enhances the current range offered in the field of ophthalmology. With the incorporation of CIMERLI®, Sandoz reaffirms its dedication to the biosimilar market, taking a substantial leap toward the objective of leading the way in expanding access to essential, more cost-effective medications across the United States.
Keren Haruvi, the head of Sandoz operations in North America, expressed satisfaction with the introduction of another significant addition to the growing collection of biosimilar products under Sandoz. This expansion enhances the current range offered in the field of ophthalmology. With the incorporation of CIMERLI®, Sandoz reaffirms its dedication to the biosimilar market, taking a substantial leap toward the objective of leading the way in expanding access to essential, more cost-effective medications across the United States.
Sandoz eagerly anticipates the opportunity to offer a broader spectrum of medicinal choices to American individuals dealing with visual impairments and deterioration. By acquiring the CIMERLI®* enterprise from Coherus, Sandoz is positioned to enhance its ophthalmic suite of services, paving the way for the introduction of future medications.
CIMERLI®* is a biosimilar available in injectable forms of 0.3 mg and 0.5 mg, sanctioned by the FDA to match the original LUCENTIS® (ranibizumab injection). It is prescribed for several retinal conditions, including the wet form of age-related macular degeneration, diabetic retinal swelling, and the swelling occurring after a blockage in the retinal vein.
As a biologic medication in the anti-VEGF category, CIMERLI® plays a crucial role in assisting individuals with retinal issues in maintaining or improving their sight. The FDA gave its approval to CIMERLI®* in August 2022, validating its biosimilarity to the original product based on the stringent criteria of safety, efficacy, and quality. When it was released in October 2022, it became the uniquely approved biosimilar that was deemed interchangeable with LUCENTIS® for all its prescribed uses.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
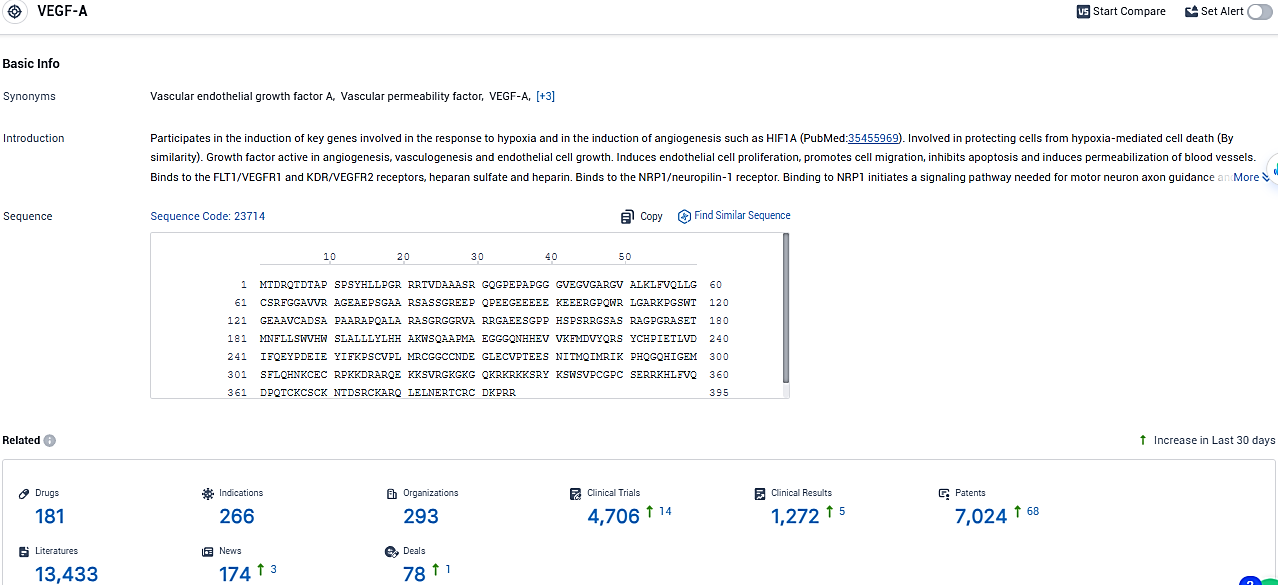
According to the data provided by the Synapse Database, As of January 26, 2024, there are 181 investigational drugs for the VEGF-A target, including 266 indications, 293 R&D institutions involved, with related clinical trials reaching 4706, and as many as 7024 patents.
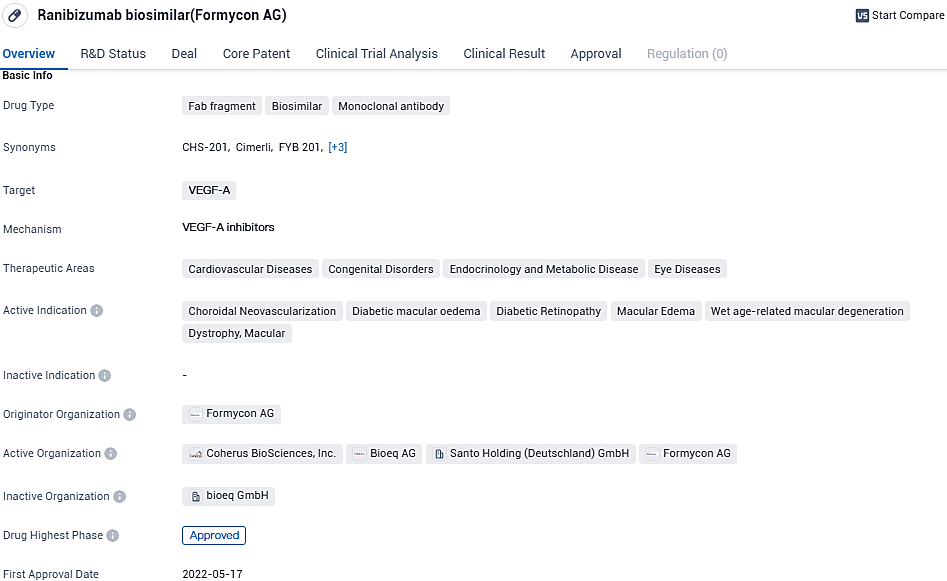
CIMERLI® is a Fab fragment, biosimilar, monoclonal antibody that targets VEGF-A. It is used in the treatment of various therapeutic areas including cardiovascular diseases, congenital disorders, endocrinology and metabolic disease, and eye diseases. It provides hope for patients suffering from various eye diseases and conditions related to cardiovascular diseases, congenital disorders, endocrinology and metabolic disease, and offers a potential alternative treatment option.