Phase 3 Study of BNT323/DB-1303 for Advanced Breast Cancer Launched by BioNTech & DualityBio
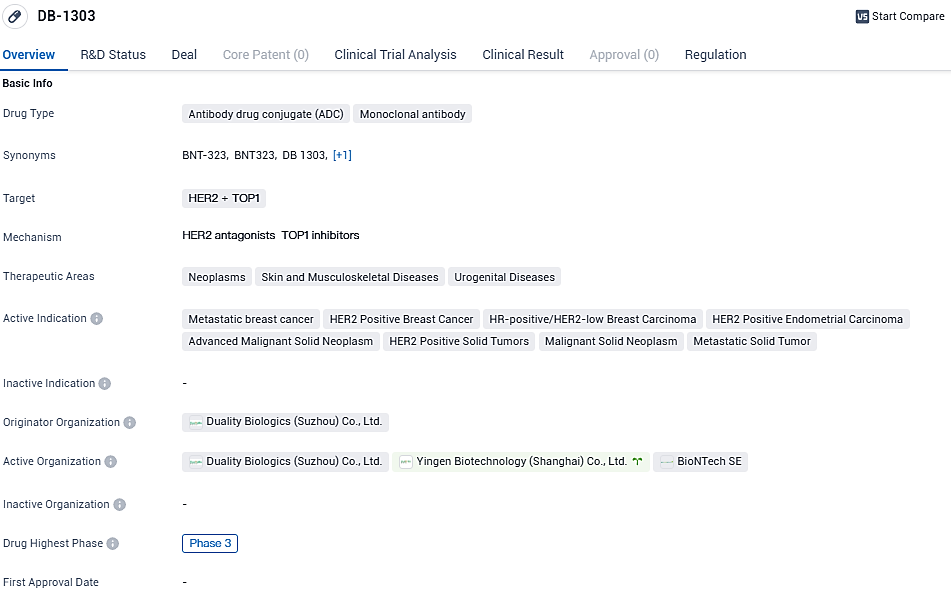
BioNTech SE, alongside Duality Biologics (Suzhou) Co., Ltd., have publicly disclosed initiation of a critical Phase 3 study involving their innovative antibody-drug conjugate known as BNT323/DB-1303. This clinical trial, designed to assess both effectiveness and tolerability, has commenced with the administration to the initial patient diagnosed with advanced HER2-positive breast carcinoma. HER2, a receptor protein found on the surface of certain cancer cells, is the specific biomarker targeted by this therapy.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Globally, breast cancer represents the most frequent cancer diagnosis and is the primary reason for fatal malignant growths in the female population. A particular breast cancer category, characterized by the presence of hormone receptors and the minimal presence of the HER2 protein on the outer membrane of the cancerous cells, embodies roughly 40 to 45 percent of those facing the advanced, metastatic stages of the disease.
Targeting HER2 has proven valuable in treating breast malignancies with moderate to high levels of HER2. Prior attempts to target HER2 in cancers expressing low levels of this protein have not been successful. Emerging research suggests that avant-garde Antibody-Drug Conjugates (ADCs) could extend the benefits of HER2-centered treatments to cancers with lesser HER2 presence.
In a widespread, multi-institutional, openly labeled, and randomized Phase 3 clinical test, the performance and tolerability of BNT323/DB-1303 will be measured against the usual chemotherapy provided to first-time chemotherapy patients who have Hormone Receptor-Positive (HR+) and HER2-low metastatic breast cancer and have shown progression following hormone therapy. The clinical test plans to include 532 participants across upward of 223 research locations on a global scale, beginning in China with subsequent expansions to the United States, Europe, and other areas.
This significant development aligns with the strategic goals of BioNTech and DualityBio to push this investigational therapy into advanced trials for cancer types with substantial unmet medical needs. The commencement of the Phase 3 trial represents a key milestone in the strategic alliance between BioNTech and DualityBio, which commenced in April 2023. This partnership is dedicated to hastening the progress of unique ADC therapies for the treatment of solid malignancies.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
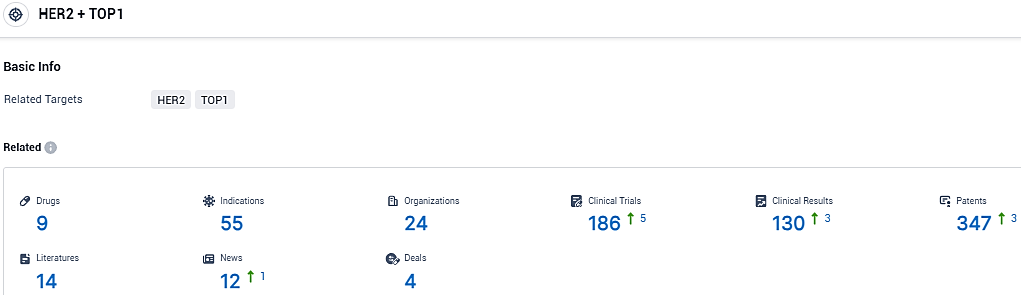
According to the data provided by the Synapse Database, As of January 26, 2024, there are 9 investigational drugs for the HER2 and TOP1 target, including 55 indications, 24 R&D institutions involved, with related clinical trials reaching 186, and as many as 347 patents.
DB-1303 shows promise as a potential treatment option for various types of cancers, particularly those associated with HER2 and TOP1. The BNT323/DB-1303 program received the Fast Track designation and Breakthrough Therapy designation from the U.S. Food and Drug Administration for the treatment of endometrial cancer in 2023. Further research and clinical trials will be necessary to determine the drug's safety and efficacy in larger patient populations.