Harpoon Therapeutics shares progress on HPN217 development with AbbVie
The clinical-stage immunotherapy firm, Harpoon Therapeutics, Inc., specializing in the generation of innovative T cell engagers, disclosed that AbbVie has informed them that the unique licensing opportunity linked to Harpoon's HPN217 program, targeting B cell maturation antigen (BCMA), as per the Development and Option Agreement of the parties.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The Agreement, set to end definitively on October 12, 2023, has provided AbbVie with the potential for a global, exclusive license regarding the HPN217 project. The program is, and will continue to, be solely in the possession of Harpoon, with the firm intending to conclude the current Phase 1 clinical trial and produce data supporting the subsequent developmental phase.
"Advanced multiple myeloma patients who have deteriorated following previous treatments, including those who have undergone prior BCMA-targeted therapies, face a considerable medical necessity which has yet to be fulfilled," stated Julie Eastland, Harpoon Therapeutics' President and Chief Executive Officer. "We intend to divulge preliminary results to the scientific populace at the forthcoming IMS conference. The initial data regarding the HPN217 investigation continues to provide optimism with an advantageous safety profile."
Harpoon has communicated the acceptance of an abstract along with a poster showcasing early data up to the inclusion of 12 mg cohorts from the dose elevation aspect of the Phase 1 investigation involving HPN217 targeted at patients suffering from relapsed/refractory multiple myeloma. This display is scheduled for September 28, 2023, at the 20th International Myeloma Society Annual Meeting set to take place in Athens, Greece.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
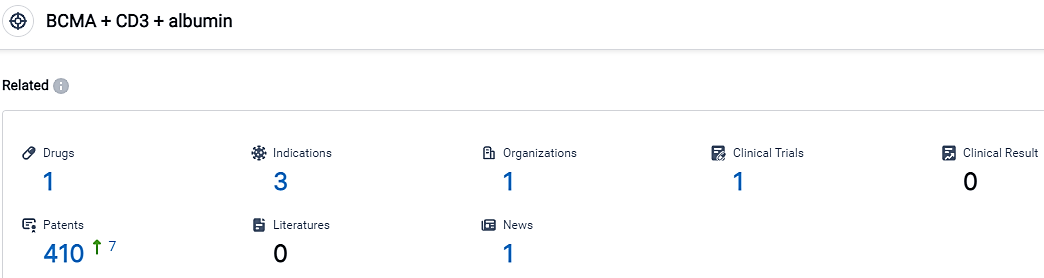
According to the data provided by the Synapse Database, As of September 19, 2023, there are 1 investigational drugs for the BCMA and CD3 and albumin target, including 3 indications,1 R&D institutions involved, with related clinical trials reaching 1,and as many as 410 patents.
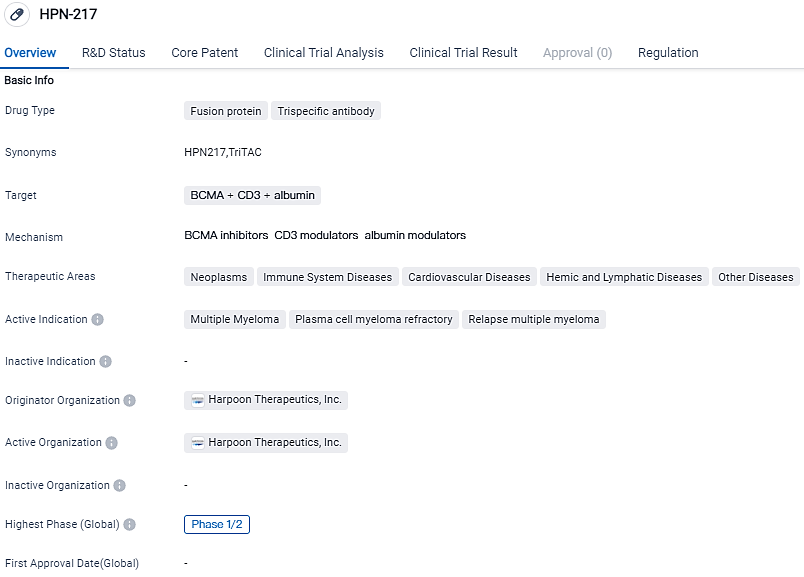
HPN-217 is a fusion protein and trispecific antibody drug being developed by Harpoon Therapeutics, Inc. It targets BCMA, CD3, and albumin and is being investigated for its potential in treating multiple myeloma and other diseases. The drug is currently in Phase 1/2 of clinical development and has been granted Fast Track and Orphan Drug designations.