UroGen has announced that the FDA has accepted its new drug application for UGN-102
UroGen Pharma Ltd. (Nasdaq: URGN), a biotechnology firm focused on creating and commercializing novel treatments for urothelial and specialized cancers, announced the acceptance of its New Drug Application (NDA) by the U.S. Food and Drug Administration (FDA) for the investigational product UGN-102 (mitomycin) intended for intravesical administration. Should it receive approval, UGN-102 would be the first drug sanctioned by the FDA for addressing low-grade intermediate-risk non-muscle invasive bladder cancer (LG-IR-NMIBC). The FDA has set a goal date of June 13, 2025, under the Prescription Drug User Fee Act (PDUFA).
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
“The acceptance of our NDA by the FDA marks a significant milestone in our efforts to introduce UGN-102 to patients,” commented Liz Barrett, President and CEO of UroGen. “If approved, UGN-102 has the potential to be the first FDA-sanctioned treatment for LG-IR-NMIBC, providing a innovative option that could broaden therapeutic choices and fulfill critical needs. There is a pressing need for groundbreaking solutions in this area, and we are committed to working closely with the FDA as we aim for a possible launch of UGN-102 in 2025.”
Dr. Mark Schoenberg, UroGen's Chief Medical Officer, noted, “The NDA for UGN-102 is supported by a strong dataset that showcases remarkable durability of response from three clinical trials along with a positive safety profile. Importantly, the ENVISION trial met its primary goal, displaying a complete response rate of 79.6% at three months post the initial instillation of UGN-102. Furthermore, the latest data from this trial indicated an estimated 82.3% duration of response at 12 months for patients who had a complete response at three months, as per the Kaplan-Meier analysis. The most frequently reported treatment-related adverse events in the ENVISION trial included dysuria, hematuria, urinary tract infection, pollakiuria, fatigue, and urinary retention.
Moreover, the safety profile identified in the ENVISION trial aligns with findings from other UGN-102 studies. We believe that, contingent on approval, UGN-102’s capacity to induce lasting complete responses and potentially lower recurrence rates while prolonging treatment-free intervals will signify a major advancement in the care of LG-IR-NMIBC.”
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
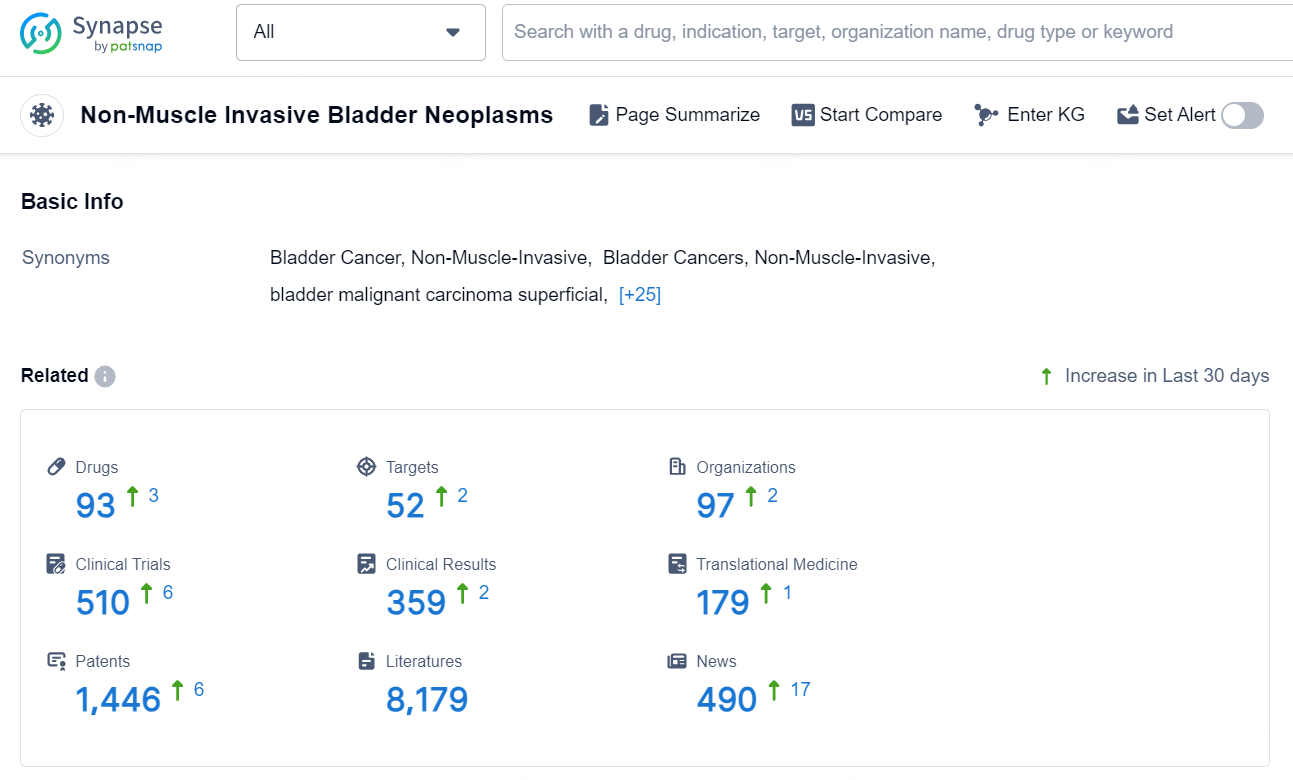
According to the data provided by the Synapse Database, As of October 18, 2024, there are 93 investigational drugs for the Non-Muscle Invasive Bladder Neoplasms, including 52 targets, 97 R&D institutions involved, with related clinical trials reaching 510, and as many as 1446 patents.
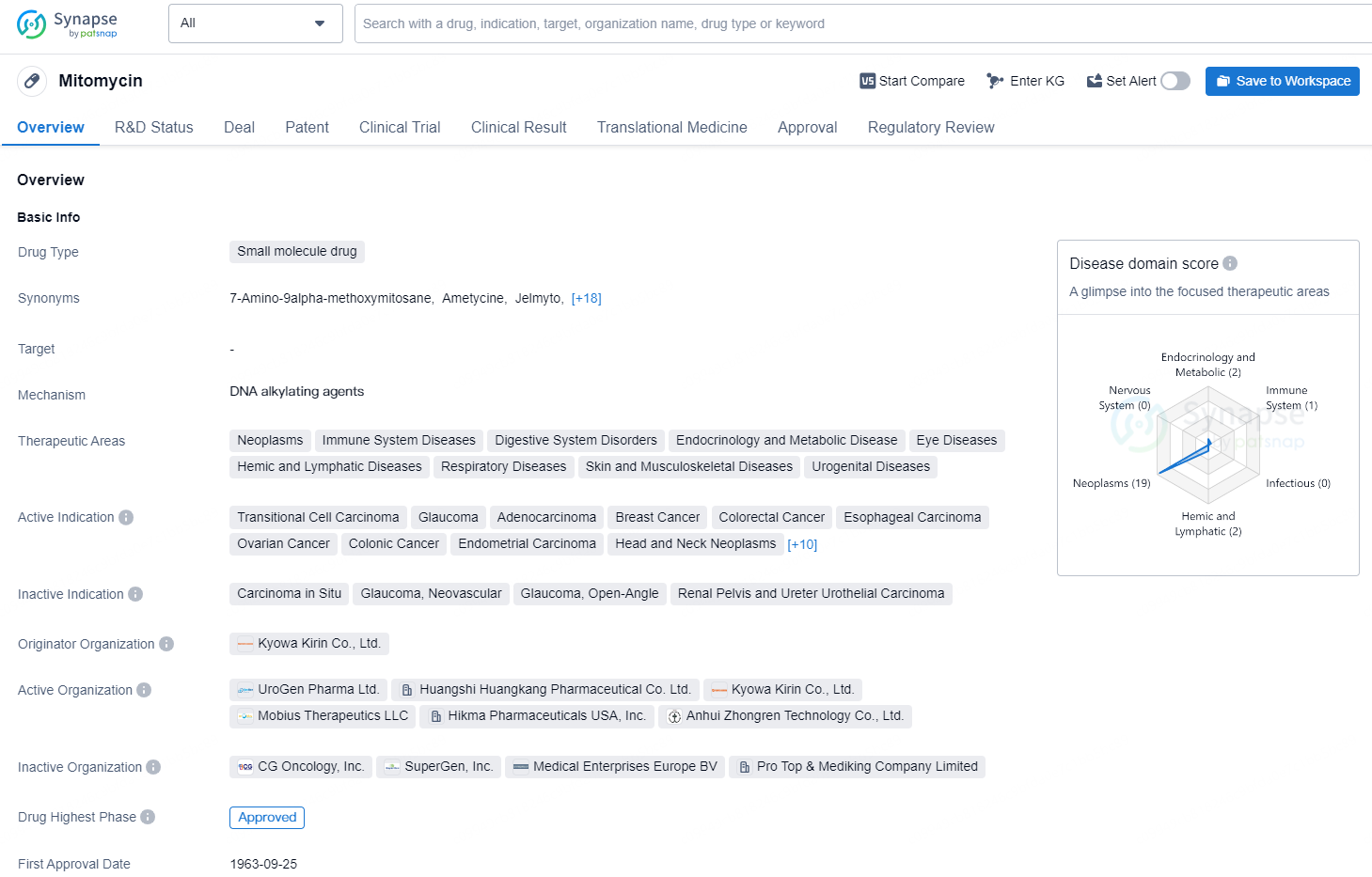
Mitomycin is a small molecule drug used in the treatment of various therapeutic areas such as neoplasms, immune system diseases, digestive system disorders, endocrinology and metabolic disease, eye diseases, hemic and lymphatic diseases, respiratory diseases, skin and musculoskeletal diseases, and urogenital diseases.