SciRhom Begins Administering Doses in First Human Trial for Its Main Development Project SR-878
SciRhom GmbH, a biopharmaceutical firm at the forefront of developing innovative iRhom2 therapeutic antibodies, has announced the initiation of participant dosing in their first clinical trial for the advanced development program SR-878. This double-blind, placebo-controlled, first-in-human trial with a single ascending dose will evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of SR-878 in a cohort of up to 48 healthy subjects. The study is being carried out in partnership with the Department of Clinical Pharmacology at the Medical University of Vienna in Austria. Results from the trial are anticipated to be available in the second half of 2025.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Launching the inaugural clinical trial utilizing our iRhom2-targeting strategy marks a pivotal milestone and significant value transition for the organization. Our aim is to validate the positive safety profile of SR-878 observed in preclinical research and to rapidly move towards showcasing the clinical advantages of our innovative approach in patients during the upcoming Phase 2 studies," stated Dr. Jürgen Reess, Chief Medical Officer of SciRhom.
Dr. Jan Poth, Managing Director and CEO of SciRhom, remarked: "Today's remarkable occasion brings us closer than ever to illustrating the groundbreaking potential of targeting iRhom2, thereby influencing the disease-related pathways regulated by TACE/ADAM17. We are thrilled to collaborate with the Department of Clinical Pharmacology at the Medical University of Vienna for this initial human study, as their expertise will be essential in laying a strong groundwork for potentially numerous follow-up studies."
Through its SR-878 initiative, SciRhom aims to introduce a novel treatment paradigm for autoimmune disorders and possibly other conditions by effectively blocking multiple pro-inflammatory and disease-inducing pathways. The precise targeting of iRhom2 is anticipated to yield enhanced effectiveness compared to existing therapies, while maintaining the critical roles associated with its interaction partner TACE/ADAM17, which should aid in achieving a favorable safety profile.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
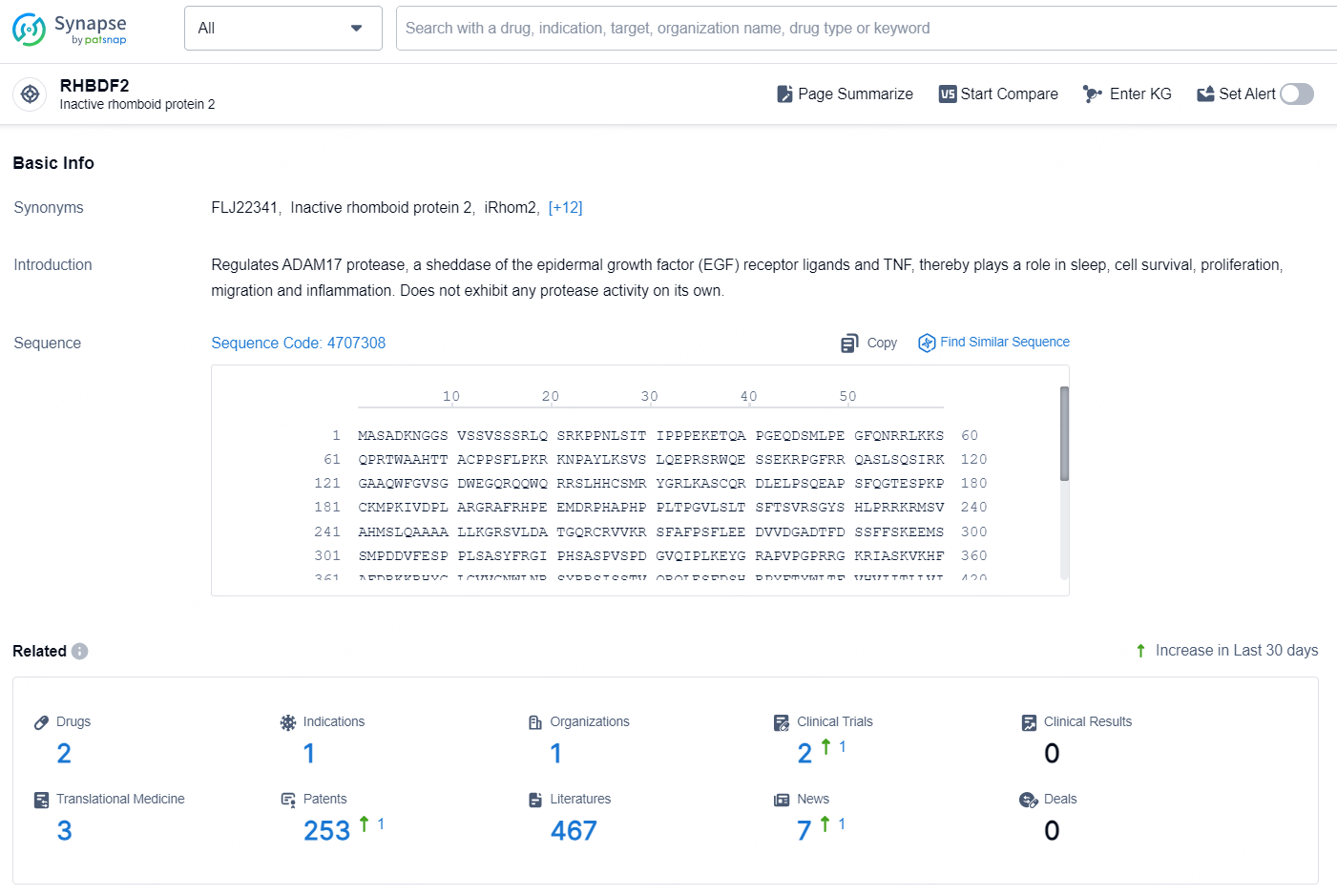
According to the data provided by the Synapse Database, As of October 18, 2024, there are 2 investigational drug for the RHBDF2 targets, including 1 indication1, 1 R&D institution involved, with related clinical trials reaching 2, and as many as 253 patents.
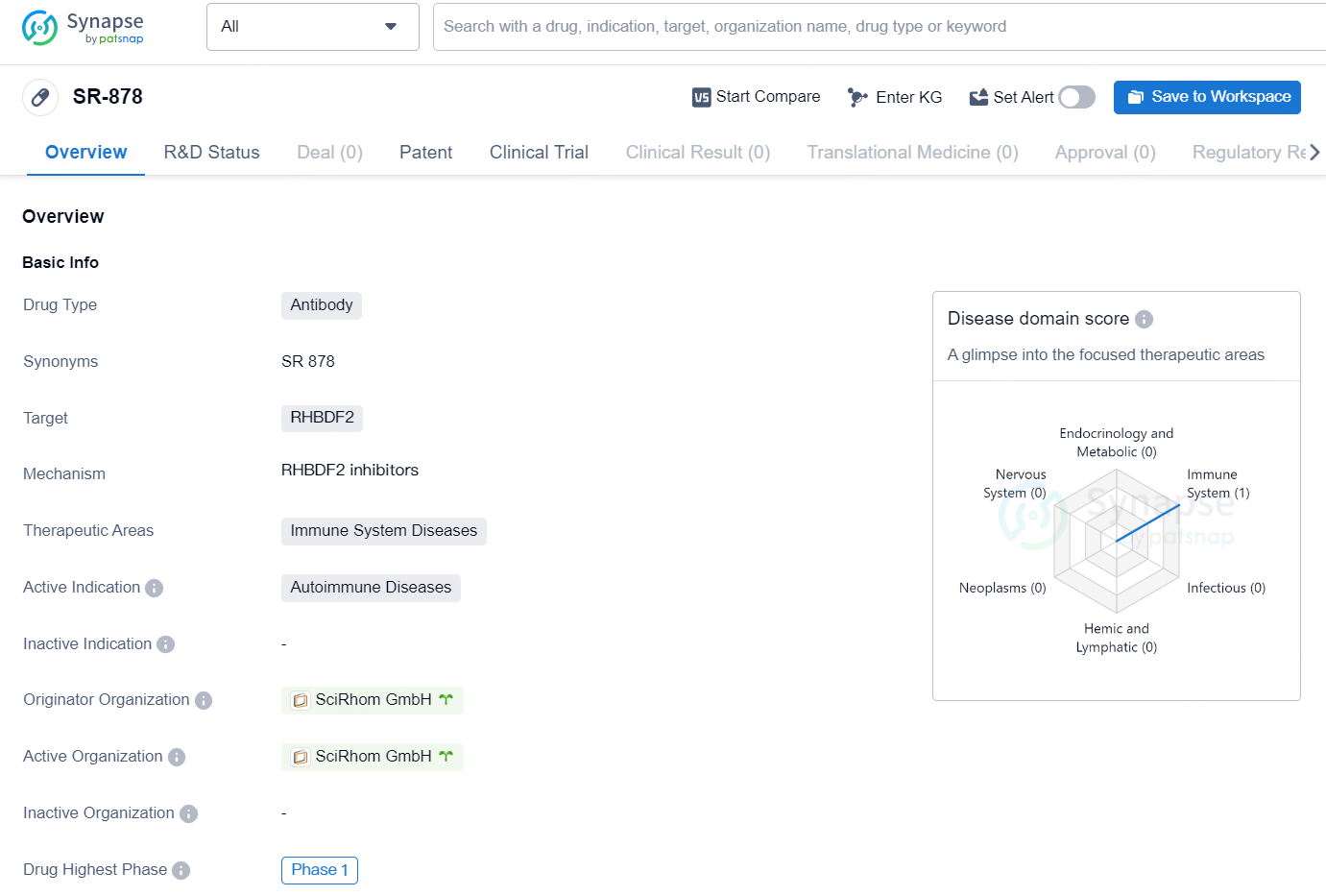
The drug [SR-878] is an antibody drug that targets the protein RHBDF2 and is being developed for the treatment of immune system diseases, specifically autoimmune diseases. The drug is currently in the Phase 1 of clinical development. The originator organization responsible for the development of this drug is SciRhom GmbH.