TEZSPIRE's Phase III WAYPOINT Success in Treating Chronic Rhinosinusitis & Nasal Polyps
Favorable topline findings from the Phase III WAYPOINT study involving individuals with chronic rhinosinusitis with nasal polyps indicated that TEZSPIRE® (tezepelumab) from AstraZeneca and Amgen resulted in a statistically significant and clinically relevant decrease in nasal polyp size and alleviated nasal congestion in comparison to the placebo group.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
The WAYPOINT study is a randomized, double-blind clinical trial designed to assess the safety and effectiveness of TEZSPIRE administered via subcutaneous injection in comparison to a placebo for adults suffering from severe chronic rhinosinusitis with nasal polyps (CRSwNP). All participants exhibited symptoms despite the use of standard treatment, which included intranasal corticosteroids (INCS).
Dr. Joseph Han, who serves as the Vice Chair of Rhinology & Endoscopic Sinus and Skull Base Surgery, as well as Allergy, Otolaryngology-Head and Neck Surgery at Eastern Virginia Medical School in the US, and is a co-primary investigator for the study, remarked: “Chronic rhinosinusitis combined with nasal polyps can severely disrupt patients’ daily lives, causing issues such as obstruction that impact smell, taste, and sleep, in addition to leading to pain and fatigue. The compelling findings from the WAYPOINT study highlight the potential of tezepelumab as a novel therapeutic option for those whose lives are affected by this challenging condition.”
Dr. Brian Lipworth, a Professor of Allergy and Pulmonology at the Scottish Centre for Respiratory Research, as well as at the Tayside Rhinology Ear, Nose, and Throat Clinic at Ninewells Hospital, University of Dundee in the UK and a co-primary investigator of the trial, stated: “Individuals diagnosed with nasal polyps frequently face substantial challenges, including multiple surgeries and the necessity for high doses of oral corticosteroids, which come with significant systemic risks. The findings regarding tezepelumab are notably significant and provide hope for new treatment alternatives that could alleviate the burden on patients and the healthcare system.”
Sharon Barr, the Executive Vice President of BioPharmaceuticals R&D, expressed enthusiasm about the encouraging outcomes from the Phase III WAYPOINT trial, indicating that patients with nasal polyps showed a marked improvement following treatment with tezepelumab. She noted that these findings underscore the first-in-class mechanism of action of tezepelumab, which targets TSLP at the initial stage of the inflammatory pathway and effectively addresses various factors contributing to epithelial-driven inflammatory diseases.
The trial confirmed that the safety and tolerability of TEZSPIRE were in line with the established profile of the medication.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
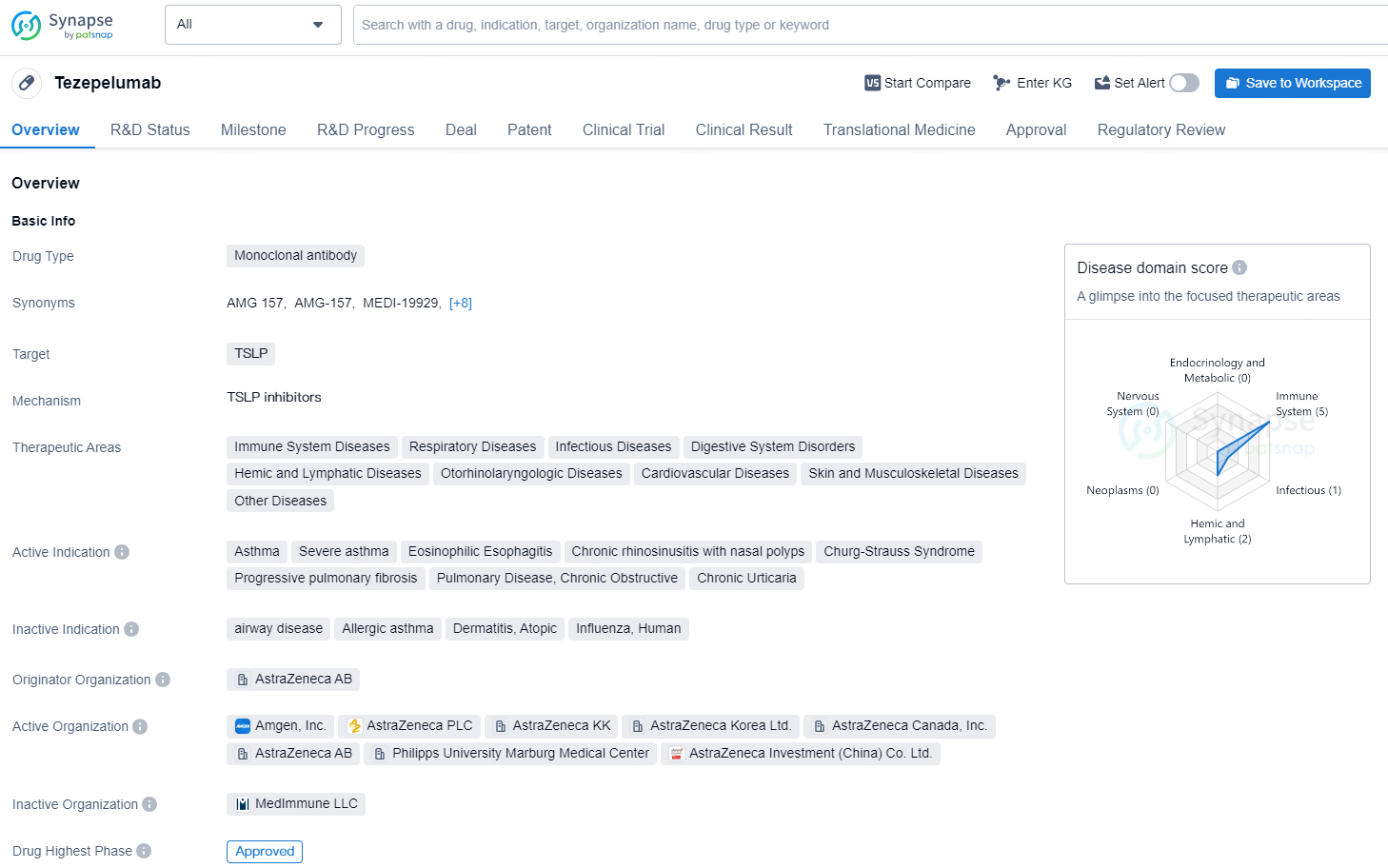
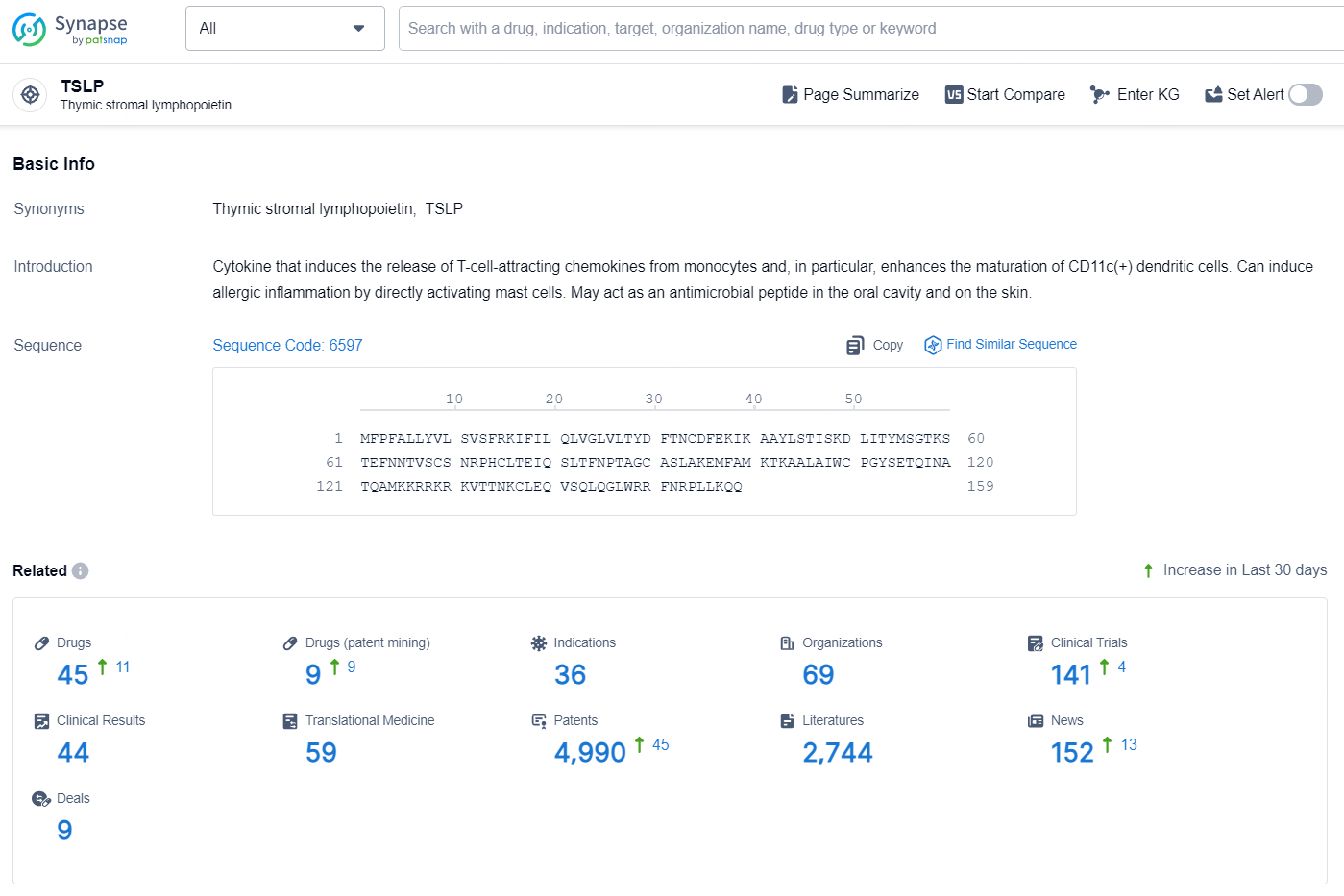
According to the data provided by the Synapse Database, As of November 11, 2024, there are 45 investigational drugs for the TSLP target, including 36 indication, 69 R&D institutions involved, with related clinical trial reaching 141, and as many as 4990 patents.
Tezepelumab is a monoclonal antibody drug developed by AstraZeneca AB, aimed at targeting TSLP. It is indicated for a wide range of therapeutic areas including immune system diseases, respiratory diseases, infectious diseases, digestive system disorders, hemic and lymphatic diseases, otorhinolaryngologic diseases, cardiovascular diseases, skin and musculoskeletal diseases, and other diseases.