IASO Bio Reveals Chinese Regulatory Green Light for Advanced MM Therapy with Equecabtagene Autoleucel
IASO Bio has disclosed official authorization from the China National Medical Products Administration for the clinical trial application of Equecabtagene Autoleucel. This innovative, entirely human BCMA-targeted CAR T-cell therapy has been green-lighted for further clinical studies focusing on participants with multiple myeloma. Specifically, the treatment is aimed at patients who have experienced a relapse or shown resistance after one to two earlier treatment regimens, including those unresponsive to the drug lenalidomide.
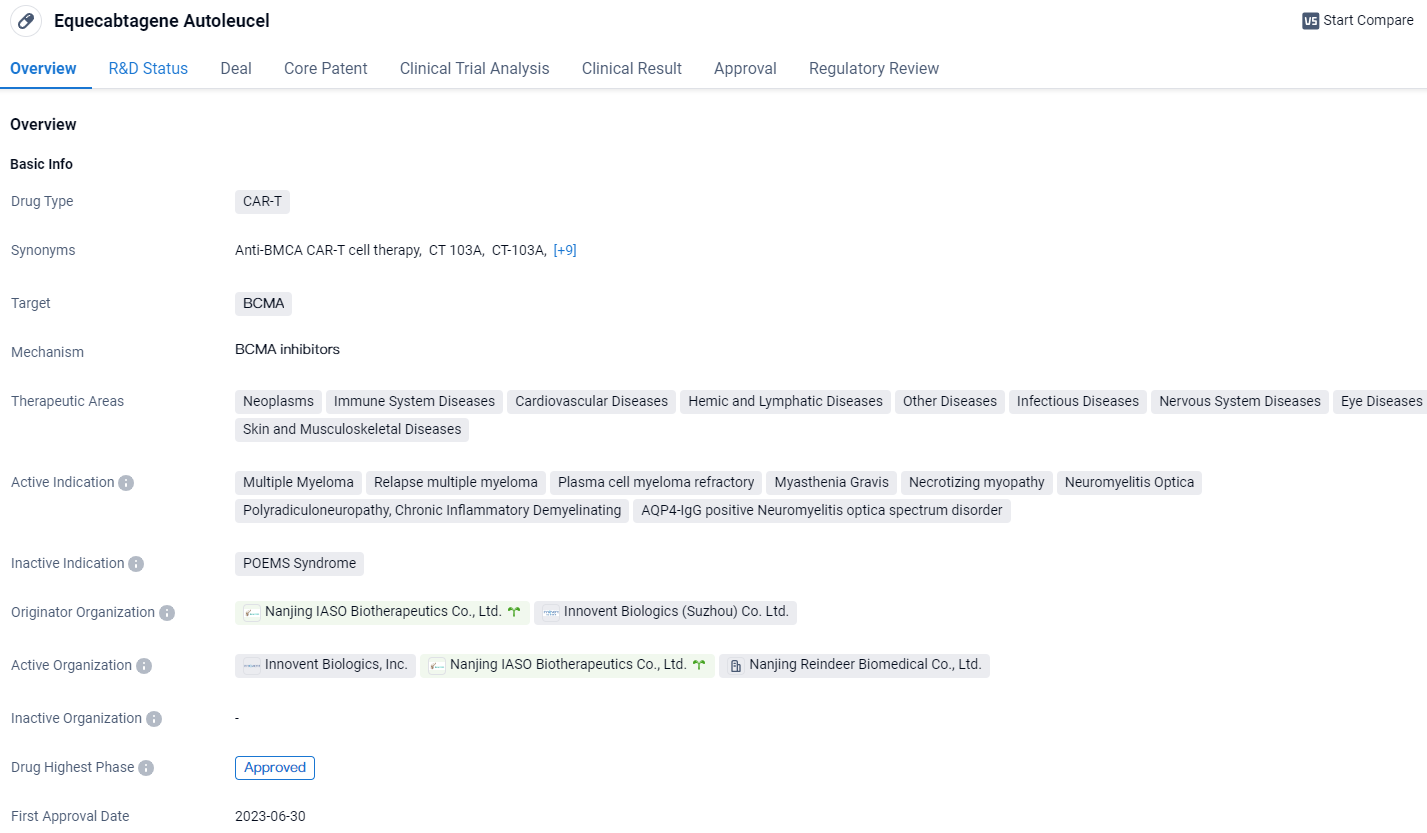
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
On June 30, 2023, the National Medical Products Administration (NMPA) gave its official clearance for the use of FUCASO (Equecabtagene Autoleucel) targeting those patient cases categorized as relapsed and/or refractory multiple myeloma after undergoing three or more different therapeutic courses that included a proteasome inhibitor and an immunomodulatory medication.
This authorization of the New Drug Application (NDA) comes in the wake of compelling study results from the key FUMANBA-1 trial, which featured a wide range of testing locations throughout China. Presenting at the 2023 session of the International Myeloma Society Annual Meeting, recent observations continuing up until December 31, 2022, showcased a 96.1% overall response rate among 103 patients assessed for efficacy, with an impressive 77.7% achieving stringent complete response or complete response.
Notably, the study detailed that among 91 patients who had no history of prior CAR-T therapy, the overall response rate (ORR) was recorded at 98.9%, 82.4% attaining stringent CR/CR, and the twelve-month stretch without disease progression was measured at 85.5%. Additionally, a remarkable 94.2% were documented as reaching minimal residual disease (MRD) negativity, while those achieving stringent CR/CR uniformly accomplished MRD negativity.
From the cohort of 105 participants, merely a single case encountered cytokine release syndrome of ≥ Grade 3, and none faced severe outcomes of ≥ Grade 3 immune effector cell-associated neurotoxicity syndrome. When examining the pharmacokinetics, it was observed that Equecabtagene Autoleucel maintained presence within patients, with gene copy numbers still detectable in 40% of subjects two years post-infusion.
IASO Bio's Ms. Jinhua Zhang, who stands as the Founder, Chairman, and CEO, expressed enthusiasm regarding the NMPA's decision to greenlight Equecabtagene Autoleucel for second- and third-line treatment in R/RMM cases. As highlighted by Zhang, this milestone signifies a major step forward in the therapeutic's development trail. Committed to kick-starting the clinical enrollment phase, their team anticipates widening the spectrum of patients who can gain from this leading-edge treatment, particularly those encountering early-stage relapse.
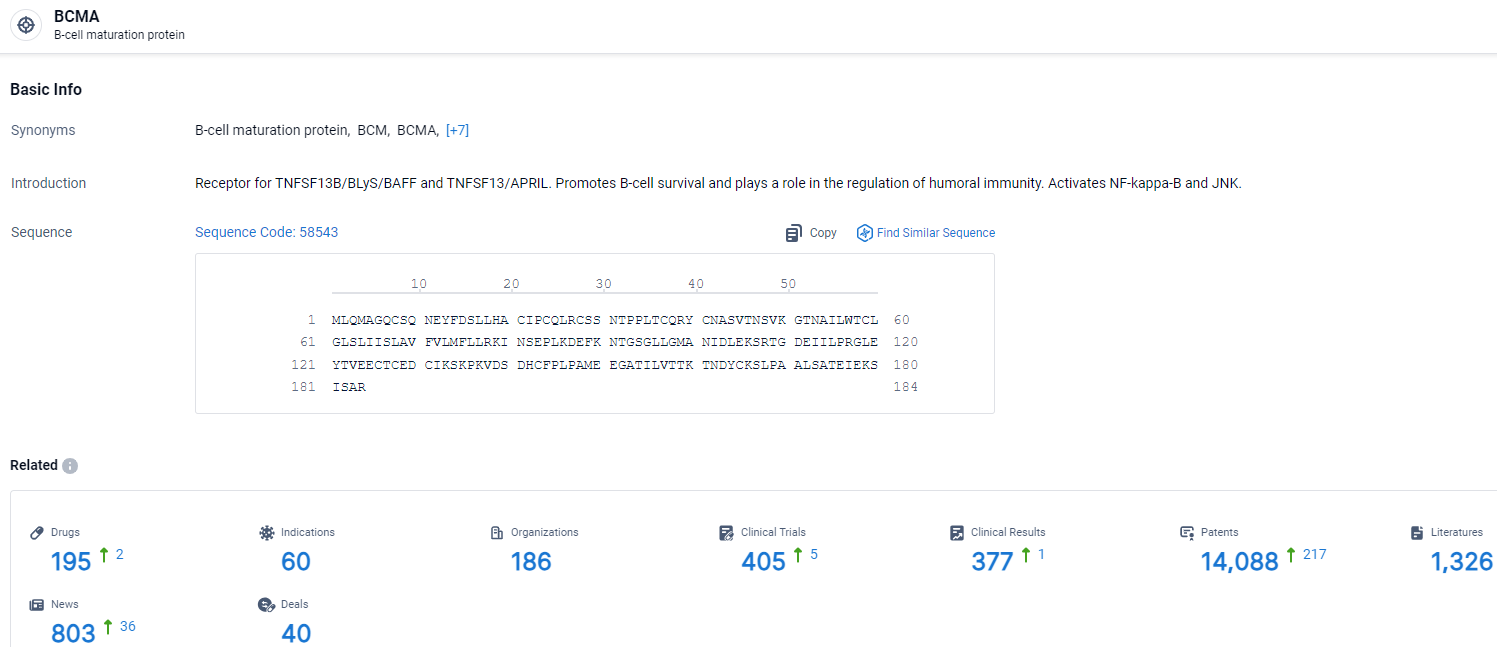
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of April 3, 2024, there are 195 investigational drugs for the BCMA target, including 60 indications, 186 R&D institutions involved, with related clinical trials reaching 405, and as many as 14088 patents.
Equecabtagene Autoleucel is a CAR-T therapy that targets BCMA and has been approved for multiple indications. It shows promise in treating various diseases across different therapeutic areas. The drug has received several regulatory designations, indicating its potential to address unmet medical needs. Equecabtagene Autoleucel represents a significant advancement in the field of biomedicine.