Overview of Genentech’s Pipeline—The renowned organization founded in 1976
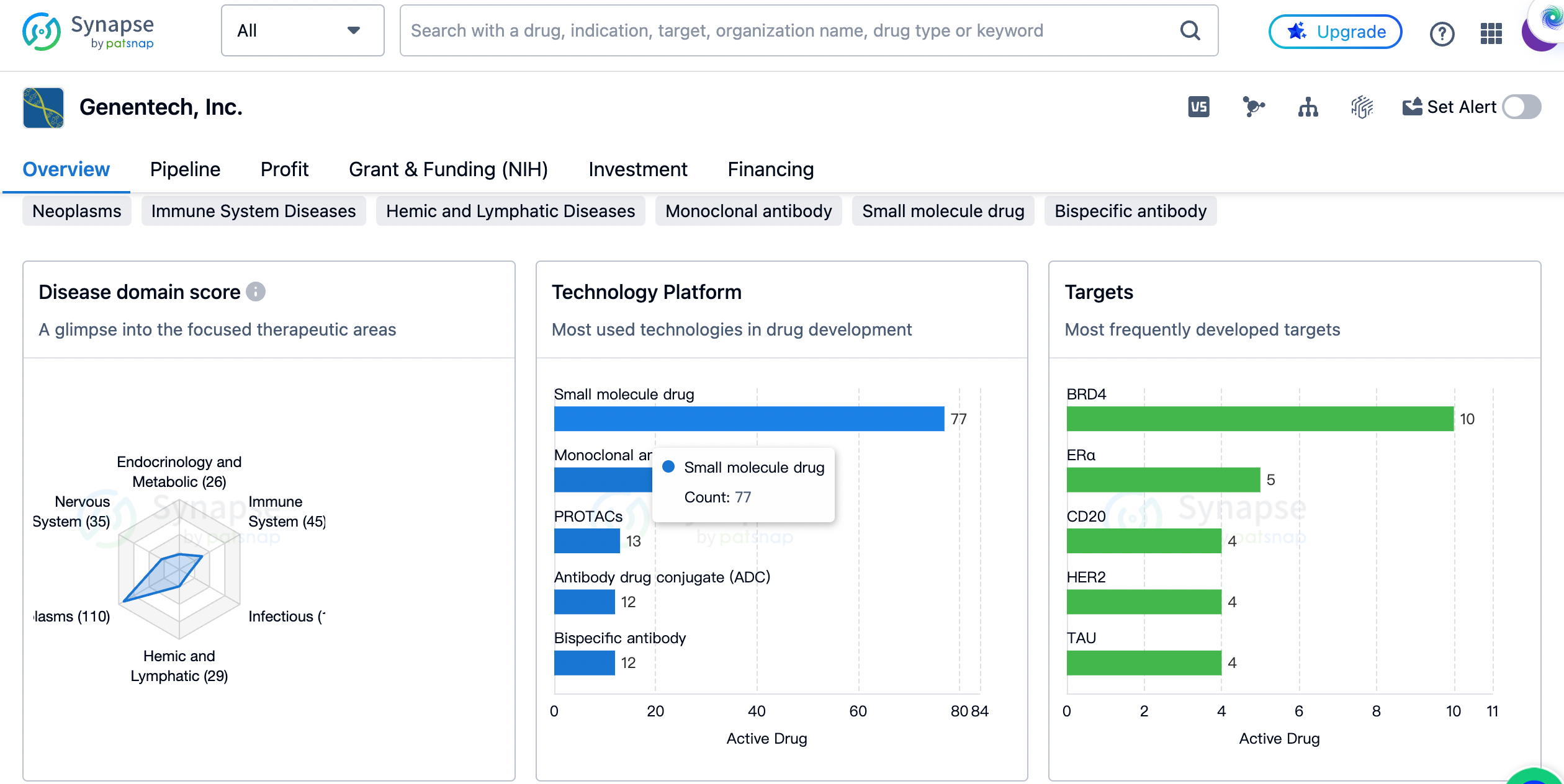
Genentech, Inc. is a renowned organization in the field of biomedicine, specializing in the development of innovative pharmaceutical products. Founded by Robert A. Swanson and Herbert Boyer in 1976 and headquartered in California, United States, Genentech has made significant contributions to the healthcare industry over the years. One of the key aspects of Genentech's business is its focus on therapeutic areas. The organization has developed drugs targeting a wide range of diseases, with a particular emphasis on neoplasms, skin and musculoskeletal diseases, and immune system diseases. These three therapeutic areas have the highest drug count, with 110, 49, and 45 drugs respectively. This indicates Genentech's commitment to addressing critical medical needs in these areas.
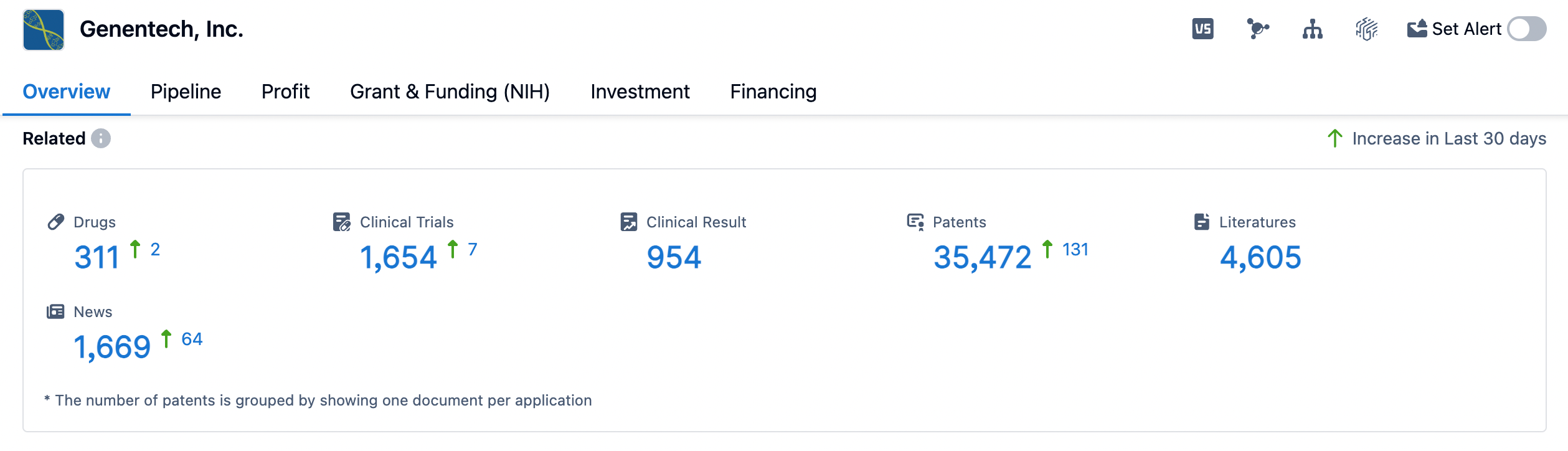
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of Genentech.
In addition to the aforementioned therapeutic areas, Genentech has also developed drugs for respiratory diseases, nervous system diseases, digestive system disorders, hemic and lymphatic diseases, and other diseases. Each of these areas has a significant drug count, highlighting Genentech's diverse portfolio and its efforts to cater to various medical conditions.
Furthermore, Genentech has shown interest in cardiovascular diseases, endocrinology and metabolic diseases, congenital disorders, infectious diseases, eye diseases, mouth and tooth diseases, and otorhinolaryngologic diseases. Although the drug count in these areas is relatively lower, it demonstrates Genentech's commitment to exploring new avenues and expanding its reach in the pharmaceutical industry.
The most frequently developed targets by genentech
Moving on to the most frequently developed targets by Genentech, several key proteins and receptors have been identified. BRD4, a protein involved in gene regulation, has the highest drug count with 10 drugs targeting it. This is followed by ERα, CD20, HER2, and TAU, each with 5, 4, 4, and 4 drugs respectively. These targets play crucial roles in various diseases, and Genentech's focus on them reflects its dedication to developing therapies that specifically address these molecular targets.
Other frequently developed targets include α-synuclein, PI3Kα, PIM, p300-CBP transcription factors, NaPi-2b, VEGF-A, ER, FGFR1 + KLB, CD22, CD20 + CD3, IgE, HER2 + Hyaluronic acid, NLRP3, and PLG. These targets are associated with a wide range of diseases, indicating Genentech's commitment to exploring different pathways and mechanisms to develop effective treatments.
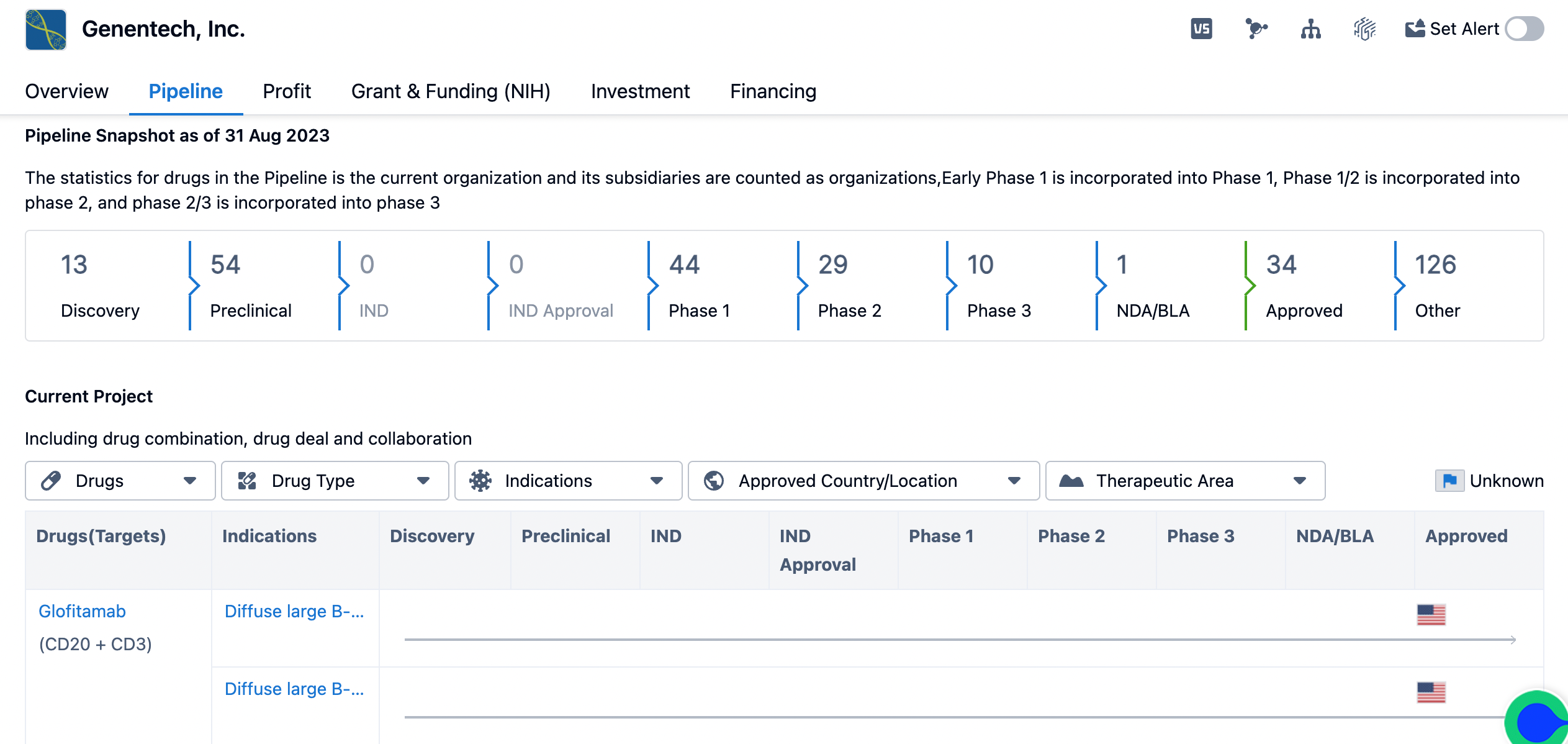
overview of genentech’s pipeline
Genentech has a robust and extensive research and development program. As of August 31, 2023, Genentech has 13 drugs in the discovery phase, 54 drugs in the preclinical phase, and 44 drugs in phase 1 of clinical trials. This demonstrates Genentech's commitment to continuously exploring new drug candidates and advancing them through the various stages of development.
Furthermore, Genentech has 29 drugs in phase 2 of clinical trials, indicating that these candidates have shown promising results in early-stage trials and are being further evaluated for their safety and efficacy. Additionally, Genentech has 10 drugs in phase 3 of clinical trials, which suggests that these candidates are in advanced stages of development and are being tested on a larger patient population to gather more comprehensive data.
Genentech also has one drug in the NDA/BLA (New Drug Application/Biologics License Application) stage, indicating that it has submitted the necessary documentation to regulatory authorities for approval. This stage is crucial as it determines whether the drug meets the required standards of safety and efficacy for commercialization. Moreover, Genentech has 34 drugs that have received approval, indicating successful completion of the regulatory process and authorization for commercialization. This demonstrates Genentech's track record of bringing innovative therapies to market and providing patients with access to much-needed treatments.
In addition to the drugs in various stages of development, Genentech has a significant number of drugs categorized as "Other" in its pipeline. These drugs may be in early research stages, undergoing formulation development, or being evaluated for potential partnerships or licensing opportunities. The high count of drugs in this category reflects Genentech's commitment to continuously exploring new therapeutic options and expanding its portfolio.
Overall, Genentech, Inc. has established itself as a prominent player in the pharmaceutical industry, with a strong focus on developing innovative therapies for a wide range of diseases. Its extensive pipeline, diverse therapeutic areas, and frequent development of drugs targeting specific molecular targets demonstrate its commitment to addressing unmet medical needs and improving patient outcomes. With its rich history and ongoing research and development efforts, Genentech is poised to continue making significant contributions to the field of biomedicine.